224452-66-8
| Name | Retapamulin |
|---|---|
| Synonyms |
Rebapamulin
Altabax Altargo (1S,2R,3S,4S,6R,7R,8R,14R)-3-Hydroxy-2,4,7,14-tetramethyl-9-oxo-4-vinyltricyclo[5.4.3.0]tetradec-6-yl {[(3-exo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl]sulfanyl}acetate MFCD11045316 Acetic acid, 2-[[(3-exo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl]thio]-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten-8-yl ester Retapamulin |
| Description | Retapamulin(SB-275833) is a topical antibiotic, which binds to both E. coli and S. aureus ribosomes with similar potencies with Kd of 3 nM.IC50 Value: 3 nM(Kd, E.coli)Target: AntibacterialRetapamulin is a topical antibiotic developed by GlaxoSmithKline. Retapamulin(SB-275833) is the first drug in the new class of pleuromutilin antibiotics to be approved for human use.Retapamulin(SB-275833) is marketed as an ointment under the brand names Altabax and Altargo. Retapamulin(SB-275833) is useful for Antibiotics. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 594.9±50.0 °C at 760 mmHg |
| Molecular Formula | C30H47NO4S |
| Molecular Weight | 517.763 |
| Flash Point | 313.6±30.1 °C |
| Exact Mass | 517.322571 |
| PSA | 92.14000 |
| LogP | 5.45 |
| Vapour Pressure | 0.0±3.8 mmHg at 25°C |
| Index of Refraction | 1.571 |
| Storage condition | 2~8℃ |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933990090 |
|
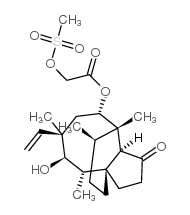
~81% 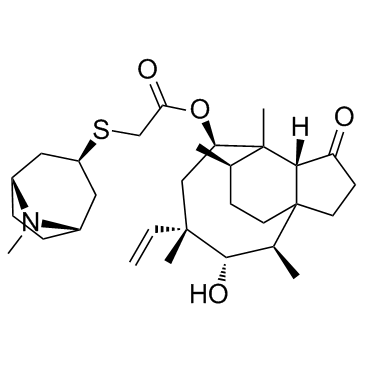
224452-66-8 |
| Literature: Hedvati, Lilach; Gilboa, Eyal; Avhar-Maydan, Sharon; Shachan-Tov, Sharona Patent: US2009/149655 A1, 2009 ; Location in patent: Page/Page column 3; 5; 6 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |