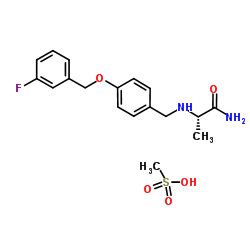
202825-46-5
| Name | Safinamide mesylate salt |
|---|---|
| Synonyms |
(S)-2-[[4-[(3-Fluorobenzyl)oxy]benzyl]amino]propanamide
N-{4-[(3-Fluorobenzyl)oxy]benzyl}-L-alaninamide methanesulfonate (1:1) Propanamide, 2-[[[4-[(3-fluorophenyl)methoxy]phenyl]methyl]amino]-, (2S)-, methanesulfonate (1:1) (2S)-2-[[4-[(3-fluorophenyl)methoxy]phenyl]methylamino]propanamide,methanesulfonic acid PNU-151774E,NW-1015 Safinamide mesylate (S)-2-(4-(3-fluorobenzyloxy)benzylamino)propanamide,methanesulfonate (S)-(+)-2-[[4-(3-Fluorobenzoxy)benzyl]amino]propanamide FCE-28073(R-isomer),PNU-151774E,NW-1015 |
| Description | Safinamide mesylate mesylate (FCE 26743 mesylate; EMD 1195686 mesylate) is a potent, selective, and reversible monoamine oxidase B (MAO-B) inhibitor (IC50=0.098 µM) over MAO-A (IC50=580 µM)[1]. Safinamide mesylate also blocks sodium channels and modulates glutamate (Glu) release, showing a greater affinity at depolarized (IC50=8 µM) than at resting (IC50=262 µM) potentials. Safinamide mesylate has neuroprotective and neurorescuing effects and can be used for the study of parkinson disease, ischemia stroke etc.al[2][3]. |
|---|---|
| Related Catalog | |
| Target |
MAO-B:98 nM (IC50) MAO-A:580 nM (IC50) |
| In Vitro | Safinamide mesylate (1-300 µM) reduces the amplitude of the peak sodium currents in a concentration-dependent manner. When currents are stimulated to a Vtest of +10 mV from a Vh of -110 mV, the IC50 value was 262 µM. When the holding potential is depolarized to -53 mV, the inhibitory effect of Safinamide mesylate with a lower IC50 value (8 µM) in rat cortical neurons[1]. |
| In Vivo | Safinamide mesylate (intraperitoneal injection; 90 mg/kg; once daily; 14 days) treatment prior to MCAO significantly ameliorates MCAO-caused cerebral infarction volume, neurological deficit, disruption of the brain-blood barrier (BBB), and impairs expression of tight junction protein occludin and ZO-1 in mice[3]. Safinamide mesylate (intraperitoneal injection; 5 mg/kg, 15 mg/kg and 30 mg/kg) dose dependently inhibits the veratridine-induced GABA release and Glu release in vivo. At the dose 30 mg/kg, Safinamide mesylate prevents the effect of veratridine both on Glu (treatment F1,8=1.31; time×treatment interaction F8,64=2.4) and GABA (treatment F1,8=4.04; time F8,64=3.76, time×treatment interaction F8,64=2.83) release. Safinamide mesylate causes a slight, albeit not significant, reduction of veratridine-stimulated Glu release at 0.5 mg/kg and full inhibition at 5 and 15 mg/kg in rat[3]. |
| References |
| Boiling Point | 476.7ºC at 760 mmHg |
|---|---|
| Melting Point | 210° (dec) |
| Molecular Formula | C18H23FN2O5S |
| Molecular Weight | 398.449 |
| Flash Point | 242.1ºC |
| Exact Mass | 398.131165 |
| PSA | 127.10000 |
| LogP | 4.04410 |
| Vapour Pressure | 2.98E-09mmHg at 25°C |
| Symbol |



GHS05, GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H318-H400 |
| Precautionary Statements | P273-P280-P301 + P310-P305 + P351 + P338 |
| Hazard Codes | C |
| RIDADR | UN 2811 6.1 / PGIII |
| HS Code | 2924299090 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |