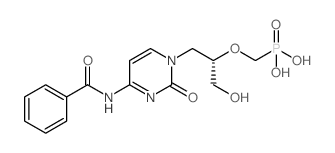
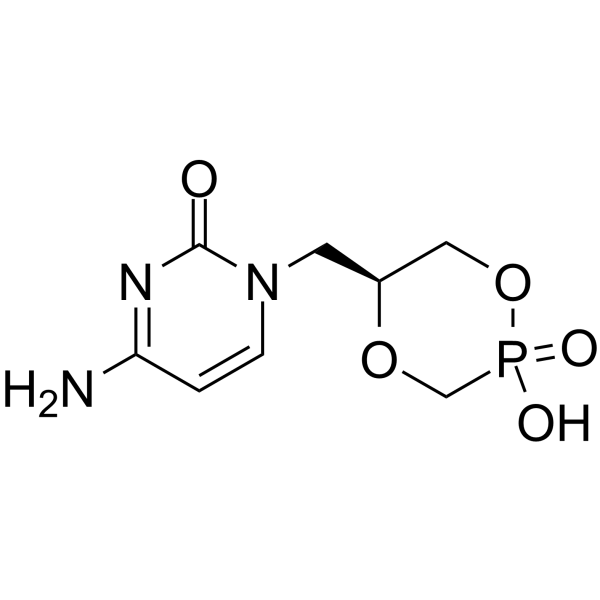
113852-37-2
| Name | cidofovir anhydrous |
|---|---|
| Synonyms |
Cidovir (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine HPMPC Vistide
Phosphonic acid, [[(1S)-2-(4-amino-2-oxo-1(2H)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]- ({[(2S)-1-(4-Amino-2-oxo-1(2H)-pyrimidinyl)-3-hydroxy-2-propanyl]oxy}methyl)phosphonic acid Vistide ({[(2S)-1-(4-Amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxypropan-2-yl]oxy}methyl)phosphonic acid Cidofovir (S)-(3-(4-amino-2-oxopyrimidin-1(2H)-yl)-1-hydroxypropan-2-yloxy)methylphosphonic acid (S)-[[2-(4-Amino-2-oxo-1(2H)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]phosphonic Acid MFCD00866936 Cidofovir hydrate GS-504 |
| Description | Cidofovir is an anti-CMV drug which can suppress CMV replication by selective inhibition of viral DNA polymerase and therefore prevention of viral replication and transcription.IC50 Value:Target: CMV DNA polymerasein vitro: The minimum concentrations of (S)-HPMPC required to inhibit CMV plaque formation by 50% was microgram/ml. The selectivity indices of (S)-HPMPC, as determined by the ratio of the 50% inhibitory concentration for cell growth to the 50% inhibitory concentration for plaque formation for CMV (AD-169 strain), was 1,500 [1]. The time course of uptake of HPMPC into Vero cells was linear between 10 and 75 min and proportional to the concentration in the medium from 10(-6) to 10(-2) M. HPMPC uptake was temperature sensitive and the rate of uptake was considerably lower at 27 degrees than at 37 degrees and almost totally inhibited at 4 degrees [2]. in vivo: Levels of cidofovirin serum following intravenous infusion were dose proportional over the dose range of 1.0 to 10.0 mg/kg of body weight and declined biexponentially with an overall mean +/- standard deviation terminal half-life of 2.6 +/- 1.2 h (n = 25). Approximately 90% of the intravenous dose was recovered unchanged in the urine in 24 h. The overall mean +/- standard deviation total clearance of the drug from serum (148 +/- 25 ml/h/kg; n = 25) approximated renal clearance (129 +/- 42 ml/h/kg; n = 25), which was significantly higher (P < 0.001) than the baseline creatinine clearance in the same patients (83 +/- 21 ml/h/kg; n = 12) [3]. Positive CMV urine cultures reverted to negative in 2 of 8 patients receiving doses of < or = 1.5 mg/kg twice weekly and 11 of 13 patients receiving higher doses. Cidofovir has in vivo anti-CMV activity demonstrated by prolonged clearing of CMV viruria, although this observation is tempered by the fact that clearance of viremia could not be demonstrated [4].Toxicity: Patients receiving 0.5 or 1.5 mg/kg twice weekly experienced no serious toxicity. The first two patients who received 5 mg/kg twice weekly developed glycosuria and 2+ proteinuria. Subsequent patients received concomitant probenecid to attempt to ameliorate renal toxicity [4].Clinical trial: FDA approved drug |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 609.5±65.0 °C at 760 mmHg |
| Melting Point | 260ºC |
| Molecular Formula | C8H14N3O6P |
| Molecular Weight | 279.187 |
| Flash Point | 322.4±34.3 °C |
| Exact Mass | 279.062012 |
| PSA | 157.71000 |
| LogP | -3.37 |
| Vapour Pressure | 0.0±4.0 mmHg at 25°C |
| Index of Refraction | 1.656 |
| Storage condition | room temp |
| Precursor 6 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![(S)-1-[3-Hydroxy-2-[(diethylphosphonyl)methoxy]propyl]cytosine structure](https://image.chemsrc.com/caspic/270/120362-35-8.png)
![(S)-1-[3-(Benzyloxy)-2-[(diethylphosphonyl)methoxy]propyl]cytosine structure](https://image.chemsrc.com/caspic/346/120362-33-6.png)