10262-69-8
| Name | maprotiline |
|---|---|
| Synonyms |
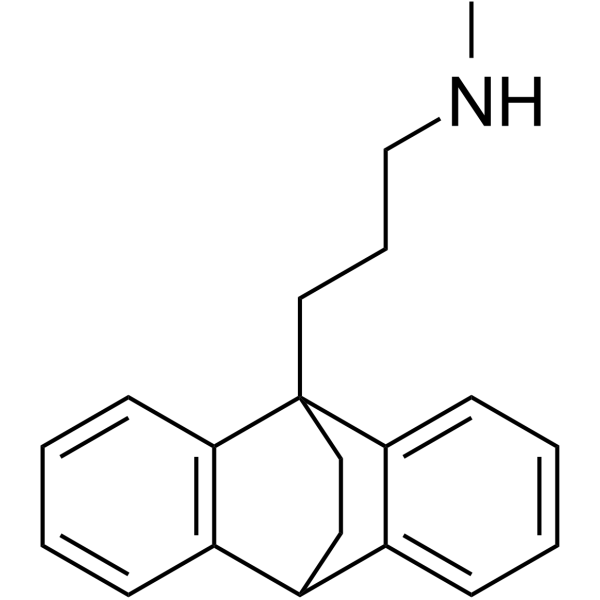
3-(9,10-Dihydro-9,10-ethanoanthracen-9-yl)-N-methylpropan-1-amine
Deprilept Dibencycladine N-methyl-9,10-ethanoanthracene-9(10H)-propanamine Maprotilina Maprotylina [Polish] Maprotilinum MFCD00661057 Maprotilin Maprotilinum [INN-Latin] EINECS 233-599-4 9-[3-(methylamino)propyl]-9,10-dihydro-9,10-ethanoanthracene Maprotilina [INN-Spanish] Maprotylina [14C]-Maprotiline |
| Description | Maprotiline is a highly selective noradrenergic reuptake blocker, has strong antidepressant efficacy. Maprotiline induces cancer cells apoptosis by targeting ERK signaling pathway and CRABP1. Maprotiline restrains cell proliferation and metastasis, exhibits anticancer effect[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Maprotiline (10 μM) enhances the sensitivity of HCC cells to sorafenib (2 μM) and induces apoptosis[2]. Maprotiline (0, 10, or 20 μM for 72 h) works on ERK pathway and inhibits phosphorylation of SREBP2 in HepG2 and Huh7 cells[2]. Maprotiline may targets CRABP1 and regulate cholesterol biosynthesis in HCC cells[2]. Cell Invasion Assay[2] Cell Line: The human HCC cell lines Huh7 and HepG2 Concentration: 0, 10, 20 μM Incubation Time: 24 hours Result: Restrained HCC cells migration with inhibition of epithelial-mesenchymal transition (EMT). Cell Viability Assay[2] Cell Line: The human HCC cell lines Huh7 and HepG2 Concentration: 0, 10, 20 μM Incubation Time: 0, 24, 48, 72, 96, 120 hours Result: Triggered cell apoptosis and inhibited the cell viability of Huh7 and HepG2 cells in a dose- and time-dependent manner. Western Blot Analysis[2] Cell Line: The human HCC cell lines Huh7 and HepG2 Concentration: 0, 10, 20 μM Incubation Time: 72 hours Result: Inhibited cholesterol biosynthesis in HCC Cells. |
| In Vivo | Maprotiline (intraperitoneal injection; 3, 10, or 30 mg/kg) combinds with the synthetic cannabinoid WIN 55,212-2 and effectively reduces neuropathic pain[1]. Maprotiline (intraperitoneal injection; 0, 20, or 40 mg/kg; twice a week; 3 weeks) shows low toxicity and side effects on the organs, immune system and hematopoietic function[2]. Maprotiline (intraperitoneal injection; 0, 20, or 40 mg/kg; twice a week; 3 weeks) restrains cholesterol biosynthesis to inhibit growth and metastasis of HCC cells by interacting with CRABP1[2]. Animal Model: Male Balb-c mice (25–30 g)[1] Dosage: 3, 10, 30 mg/kg Administration: Intraperitoneal injection; evaluation 30 minutes after treatment Result: Attenuated pain-related behaviours in neuropathic mice. Animal Model: Nude mice (BALB/C nu/nu, 4–6 weeks old, female)[2] Dosage: 40 mg/kg Administration: Intraperitoneal injection; twice a week; 3 weeks Result: Decreased the cholesterol levels in serum and tumors and suppressed the growth of Huh7-derived tumor xenografts without obvious toxic effect. |
| References |
| Density | 1.08 g/cm3 |
|---|---|
| Boiling Point | 399.6ºC at 760 mmHg |
| Molecular Formula | C20H23N |
| Molecular Weight | 277.40300 |
| Flash Point | 187.7ºC |
| Exact Mass | 277.18300 |
| PSA | 12.03000 |
| LogP | 4.60230 |
| Vapour Pressure | 1.35E-06mmHg at 25°C |
| Index of Refraction | 1.599 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | 3249 |
|---|---|
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2921499090 |
|
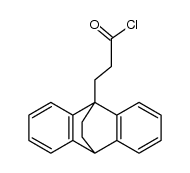
~% 
10262-69-8 |
| Literature: Helvetica chimica acta, , vol. 52, # 6 p. 1385 - 1395 |
|
~% 
10262-69-8 |
| Literature: Helvetica chimica acta, , vol. 52, # 6 p. 1385 - 1395 |
|
~% 
10262-69-8 |
| Literature: Helvetica chimica acta, , vol. 52, # 6 p. 1385 - 1395 |
|
~% 
10262-69-8 |
| Literature: Helvetica chimica acta, , vol. 52, # 6 p. 1385 - 1395 |
|
~% 
10262-69-8 |
| Literature: Helvetica chimica acta, , vol. 52, # 6 p. 1385 - 1395 |
|
~% 
10262-69-8 |
| Literature: Helvetica chimica acta, , vol. 52, # 6 p. 1385 - 1395 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2921499090 |
|---|---|
| Summary | 2921499090 other aromatic monoamines and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |