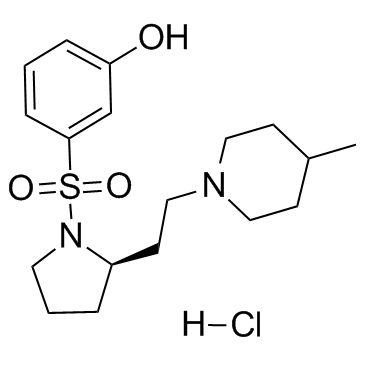
261901-57-9
| Name | (R)-3-((2-(2-(4-Methylpiperidin-1-yl)ethyl)pyrrolidin-1-yl)sulfonyl)phenol hydrochloride |
|---|---|
| Synonyms |
(R)-3-[2-[2-(4-Methylpiperidin-1-yl)ethyl]pyrrolidine-1-sulfonyl]phenol hydrochloride SB 269970A
SB-269970 (hydrochloride) |
| Description | SB269970 hydrochloride is a hydrochloride salt form of SB-269970, which is a 5-HT7 receptor antagonist with pKi of 8.3, exhibits >50-fold selectivity against other receptors.IC50 Value: 8.3 (pKi for 5-HT7) [1]Target: 5-HT7 receptorin vitro: 5-CT-stimulated adenylyl cyclase activity in guinea-pig hippocampal membranes (pEC(50) of 8.4+/-0.2) was inhibited by SB-269970-A (0.3 microM) with a pK(B) (8.3+/-0.1) in good agreement with its antagonist potency at the human cloned 5-HT(7(a)) receptor and its binding affinity at guinea-pig cortical membranes. 5-HT(7) receptor mRNA was highly expressed in human hypothalamus, amygdala, thalamus, hippocampus and testis [1]. Cortical slices were loaded with [(3)H]-5-HT and release was evoked by electrical stimulation. 5-CT inhibited the evoked release of [(3)H]-5-HT in a concentration-dependent manner. SB-269970 had no significant effect on [(3)H]-5-HT release while the 5-HT(1B) receptor antagonist, SB-224289 significantly potentiated [(3)H]-5-HT release. In addition, SB-269970 was unable to attenuate the 5-CT-induced inhibition of release while SB-224289 produced a rightward shift of the 5-CT response, generating estimated pK(B) values of 7.8 and 7.6 at the guinea-pig and rat terminal 5-HT autoreceptors respectively [2].in vivo: Acute administration of SB-269970 (1 mg/kg) or amisulpride (3 mg/kg) ameliorated ketamine-induced cognitive inflexibility and novel object recognition deficit in rats. Both compounds were also effective in attenuating ketamine-evoked disruption of social interactions [3]. Pretreatment with a dose of SB-269970 (0.5 mM) that significantly affects sleep variables antagonized the LP-44 (2.5 mM)-induced suppression of REMS and of the number of REM periods [4].Toxicity: N/AClinical trial: N/A |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C18H29ClN2O3S |
|---|---|
| Molecular Weight | 388.95200 |
| Exact Mass | 388.15900 |
| PSA | 69.23000 |
| LogP | 4.42590 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble10mg/mL, clear (warmed to 60 °C) |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2935009090 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |