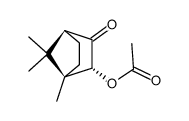
5655-61-8
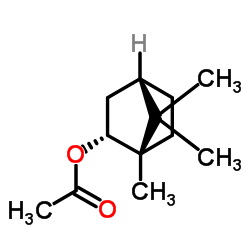
| Name | l-bornyl acetate |
|---|---|
| Synonyms |
Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S,2R,4S)-
(1S,2R,4S)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylacetat endo-bornyl acetate Bornyl ethanoate endo-(1S)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-yl acetate (-)-Bornyl acetate Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S,2R,4S)- Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, acetate, endo- L-born-2-yl acetate (1S,2R,4S)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-yl acetate endo-1,7,7-Trimethylbicyclo(2.2.1)hept-2-yl acetate Borneol Acetate EINECS 243-750-6 Bornyl Acetic ester MFCD00135942 (−)-Bornyl acetate Borneol, acetate, (1S,2R,4S)-(-)- Borneyl Acetate endo-2-Camphanyl ethanoate endo-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl acetate Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, endo- |
| Description | (-)-Bornyl acetate (L-(-)-Bornyl acetate), isolated from hyssop oil, is a less active enantiomer of (+)-Bornyl acetate. (-)-Bornyl acetate possesses antifungal activity[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | The wavy roots from seedlings exposed to (-)-bornyl acetate are significantly longer than those from seedlings exposed to ()-bornyl acetate[1]. (-)-Bornyl acetate (L-bornyl acetate), when applied individually to barley seedlings, reduced powdery mildew infection compared with controls not containing ether[2]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 223.5±0.0 °C at 760 mmHg |
| Melting Point | 29ºC |
| Molecular Formula | C12H20O2 |
| Molecular Weight | 196.286 |
| Flash Point | 84.4±0.0 °C |
| Exact Mass | 196.146332 |
| PSA | 26.30000 |
| LogP | 3.60 |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C |
| Index of Refraction | 1.480 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S24/25 |
| RIDADR | NA 1993 / PGIII |
| WGK Germany | 1 |
| Precursor 0 | |
|---|---|
| DownStream 5 | |


![[(1S,4S,5R)-4,7,7-trimethyl-2-oxo-5-bicyclo[2.2.1]heptanyl] acetate structure](https://image.chemsrc.com/caspic/287/10293-01-3.png)