82586-55-8
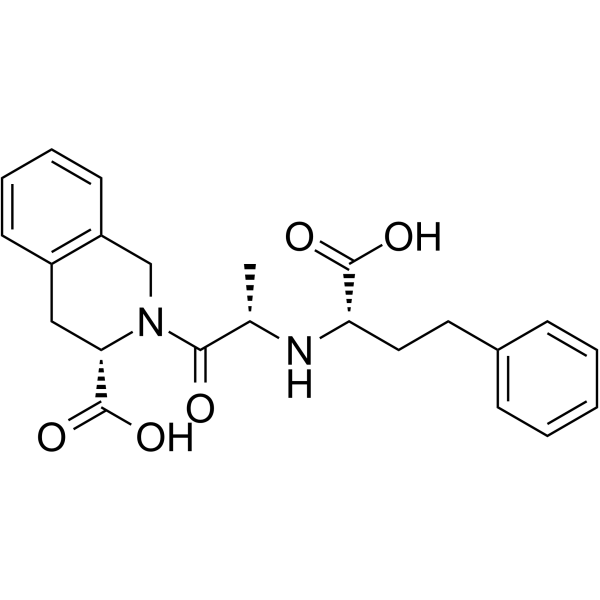
| Name | quinapril hydrochloride |
|---|---|
| Synonyms |
(3S)-2-[(2S)-2-{[(1S)-1-(Ethoxycarbonyl)-3-phenylpropyl]amino}propanoyl]-1,2,3,4-tetrahydroisochinolin-3-carbonsäurehydrochlorid
(3S)-2-{N-[(2S)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]-L-alanyl}-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid hydrochloride (1:1) (3S)-2-[(2S)-2-{[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid hydrochloride Acuitel 3-Isoquinolinecarboxylic acid, 2-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-, (3S)-, hydrochloride (1:1) ccupril Acequin quinapril HCl 2-<<1-ethoxycarbonyl)-3-phenylpropyl>amino>-1-oxopropyl>-1,2,3,4-tetrahydro-3-isoquinoline carboxylic acid monohydrochloride Conan ACCUPRIN (3S)-2-{N-[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid hydrochloride 3-isoquinolinecarboxylic acid, 2-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-, (3S)-, monohydrochloride Quinapril hydrochloride Acupril (3S)-2-{N-[(2S)-1-Ethoxy-1-oxo-4-phenylbutan-2-yl]-L-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid hydrochloride (1:1) CI-906 Acuprel MFCD00889215 ACCUPRO Korec acide (3S)-2-[(2S)-2-{[(1S)-1-(éthoxycarbonyl)-3-phénylpropyl]amino}propanoyl]-1,2,3,4-tétrahydroisoquinoléine-3-carboxylique chlorhydrate Quinapril (hydrochloride) QuinaprilHydrochloride |
| Description | Quinapril is a prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications.Target: ACEQuinapril is an angiotensin-converting enzyme inhibitor (ACE inhibitor) used in the treatment of hypertension and congestive heart failure. Quinapril is rapidly de-esterified after absorption to quinaprilat (the active diacid metabolite), a potent angiotensin converting enzyme (ACE) inhibitor. Quinapril is now firmly established as an effective and well tolerated ACE inhibitor for the treatment of patients with hypertension and congestive heart failure. Quinapril 40 mg/day also significantly reduced the incidence of ischaemic events in patients undergoing CABG in one study [1, 2]. An overview of 32 clinical trials of ACE inhibitors in heart failure showed that no significant heterogeneity in mortality was found among enalapril, ramipril, quinapril, captopril, lisinopril, benazepril, perindopril and cilazapril. Initiation of therapy with captopril, ramipril, and trandolapril at least 3 days after an acute MI resulted in all-cause mortality risk reductions of 18 to 27% [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 662ºC at 760 mmHg |
|---|---|
| Melting Point | 120-130ºC |
| Molecular Formula | C25H31ClN2O5 |
| Molecular Weight | 474.977 |
| Flash Point | 354.1ºC |
| Exact Mass | 474.192139 |
| PSA | 95.94000 |
| LogP | 3.69790 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| RTECS | NW7176000 |
|
~% 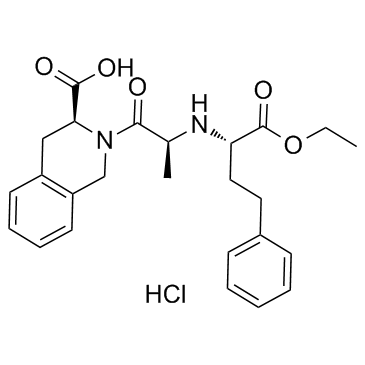
82586-55-8 |
| Literature: WO2004/54980 A1, ; Page 25;13;16 ; |
|
~% 
82586-55-8 |
| Literature: US2004/192613 A1, ; Page/Page column 5 ; |
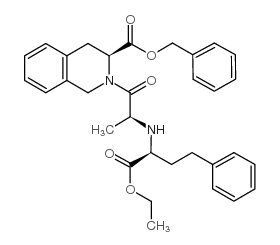
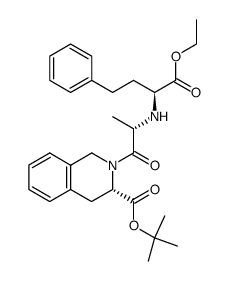
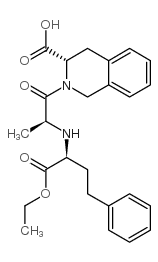
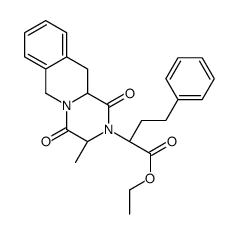
| Precursor 2 | |
|---|---|
| DownStream 4 | |
![Ethyl (3S)-2-{N-[(2S)-1-ethoxy-1-oxo-4-phenyl-2-butanyl]-L-alanyl }-1,2,3,4-tetrahydro-3-isoquinolinecarboxylate structure](https://image.chemsrc.com/caspic/334/103733-35-3.png)