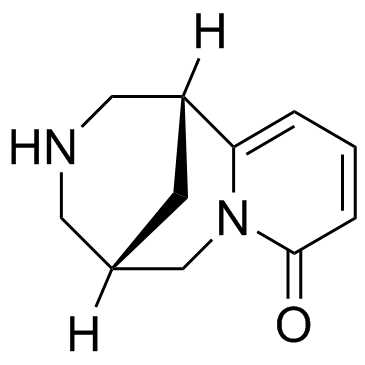
486-86-2
| Name | N-Methylcytisine |
|---|---|
| Synonyms |
N-methylcytisine
N-Methylcytisine 1,5-Methano-8H-pyrido[1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-3-methyl- 1,5-Methano-8H-pyrido[1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-3-methyl-, (1R)- (1R)-11-Methyl-7,11-diazatricyclo[7.3.1.0]trideca-2,4-dien-6-one 11-Methyl-7,11-diazatricyclo[7.3.1.0]trideca-2,4-dien-6-one 1,2,3,4,5,6-Hexahydro-3-methyl-1,5-methano-8H-pyrido[1,2-a][1,5]diazocin-8-one Caulophylline 3-methyl-1,2,3,4,5,6-hexahydro-8H-1,5-methanopyrido[1,2-a][1,5]diazocin-8-one (1R)-3-methyl-1,2,3,4,5,6-hexahydro-8H-1,5-methanopyrido[1,2-a][1,5]diazocin-8-one |
| Description | N-Methylcytisine (Caulophylline), a tricyclic quinolizidine alkaloid, exerts hypoglycaemic, analgesic and anti-inflammatory activities. N-methylcytisine is a selective ligand of nicotinic receptors of acetylcholine in the central nervous system and has a high affinity (Kd = 50 nM) to nicotinic acetylcholine receptors (nAChR) from squid optical ganglia[1][2]. |
|---|---|
| Related Catalog | |
| Target |
kd: 50 nM (nicotinic acetylcholine receptors)[2] |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 400.8±34.0 °C at 760 mmHg |
| Melting Point | 137-139ºC |
| Molecular Formula | C12H16N2O |
| Molecular Weight | 204.27 |
| Flash Point | 191.9±18.0 °C |
| Exact Mass | 204.126266 |
| PSA | 25.24000 |
| LogP | 0.46 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.616 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| RTECS | HA4400000 |
|
~99% 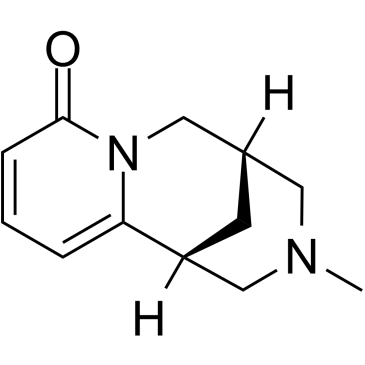
486-86-2 |
| Literature: Frigerio, Fabio; Haseler, Claire A.; Gallagher, Timothy Synlett, 2010 , # 5 p. 729 - 730 |
|
~% 
486-86-2 |
| Literature: Tsypysheva; Koval'skaya; Makara; Lobov; Petrenko; Galkin; Sapozhnikova; Zarudii; Yunusov Chemistry of Natural Compounds, 2012 , vol. 48, # 4 p. 629 - 634 Khim. Prir. Soedin., 2012 , p. 565 - 570 |