90729-43-4
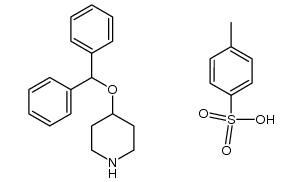
| Name | 4-(4-benzhydryloxypiperidin-1-yl)-1-(4-tert-butylphenyl)butan-1-one |
|---|---|
| Synonyms |
4-Diphenylmethoxy-1-[3-(4-tert-butylbenzoyl)propyl]piperidine
4'-tert-Butyl-4-[4-(diphenylmethoxy)piperidino]butyrophenone Ebastinum [Latin] [14C]-Ebastine carebastine 4-diphenylmethoxy-1-[3-(4-tert-butylbenzoyl)propyl]-piperidine Ebastina [Spanish] 4-[4-(Diphenylmethoxy)-1-piperidinyl]-1-[4-(2-methyl-2-propanyl)phenyl]-1-butanone Kestine LAS W-090 1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piperidin-1-yl]butan-1-one MFCD00865661 Ebastel 1-Butanone, 1-[4-(1,1-dimethylethyl)phenyl]-4-[4-(diphenylmethoxy)-1-piperidinyl]- 1-[4-(1,1-Dimethylethyl)phenyl]-4-[4-(diphenylmethoxy)-1-piperidinyl]-1-butanone Ebastin Ebastine Estivan |
| Description | Ebastine(LAS-W 090;RP64305) is a long-acting and selective H1-histamine receptor antagonist.Target: Histamine H1 ReceptorEbastine is a H1 antihistamine with low potential for causing drowsiness. Ebastine (10 mg orally) causes brain histamine H1-receptor occupation of approximately 10%, consistent with its lower incidence of sedative effect, whereas (+)-chlorpheniramine occupied about 50% of brain H1-receptors even at a low but sedative dose of 2 mg; occupancy of (+)-chlorpheniramine was correlated with plasma (+)-chlorpheniramine concentration [1]. ebastine 10 or 20 mg once daily was rapidly effective in relieving symptoms of PAR in adult and adolescent patients; additional benefits of the 20-mg dose became apparent in the longer term [2]. ebastine is an effective and generally well-tolerated treatment for allergic rhinitis and chronic idiopathic urticaria. In addition to the regular tablet formulation, ebastine is available as a FDT, providing a treatment option that is particularly convenient for patients [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 596.3±50.0 °C at 760 mmHg |
| Melting Point | 80-82°C |
| Molecular Formula | C32H39NO2 |
| Molecular Weight | 469.658 |
| Flash Point | 314.5±30.1 °C |
| Exact Mass | 469.298065 |
| PSA | 29.54000 |
| LogP | 7.79 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.590 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Statements | H413 |
|---|---|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| RIDADR | NONH for all modes of transport |
| RTECS | EL8140000 |
|
~56% 
90729-43-4 |
| Literature: Arevipharma GmbH Patent: EP2371817 A1, 2011 ; Location in patent: Page/Page column 27 ; |
|
~% 
90729-43-4 |
| Literature: WO2011/121099 A2, ; Page/Page column 42 ; |
|
~66% 
90729-43-4 |
| Literature: AREVIPHARMA GMBH; SCHICKANEDER, Christian; MCGRATH, Matthew; SCHAeFER, Juergen; SCHUBERT, Jana; JACOB, Ulrike; MAeRTENS, Welljanne Patent: WO2011/121099 A2, 2011 ; Location in patent: Page/Page column 40-41 ; |
|
~% 
90729-43-4 |
| Literature: WO2011/121099 A2, ; Page/Page column 49 ; |
|
~% 
90729-43-4 |
| Literature: WO2012/76919 A1, ; |
|
~% 
90729-43-4 |
| Literature: WO2012/76919 A1, ; |
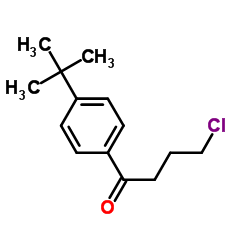
| Precursor 6 | |
|---|---|
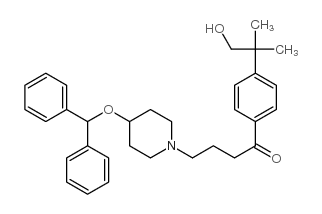
| DownStream 1 | |