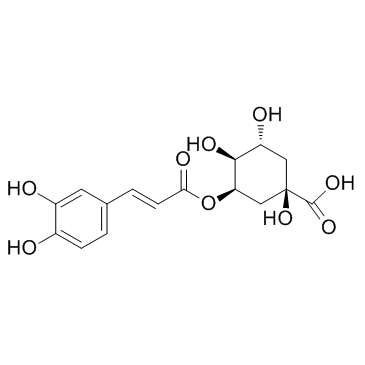
906-33-2
| Name | Neochlorogenic acid |
|---|---|
| Synonyms |
(1R,3R,4S,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxycyclohexane-1-carboxylic acid
3-{[3-(3,4-Dihydroxyphenyl)acryloyl]oxy}-1,4,5-trihydroxycyclohexanecarboxylic acid 3-[[3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]oxy] 1,4,5-trihydroxycyclohexanecarboxylic acid Cyclohexanecarboxylic acid, 3-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl]oxy]-1,4,5-trihydroxy- |
| Description | Neochlorogenic acid is a natural polyphenolic compound found in dried fruits and other plants. Neochlorogenic acid inhibits the production of TNF-α and IL-1β. Neochlorogenic acid suppresses iNOS and COX-2 protein expression. Neochlorogenic acid also inhibits phosphorylated NF-κB p65 and p38 MAPK activation. |
|---|---|
| Related Catalog | |
| Target |
p65 IL-1β TNF-α COX-2 |
| In Vitro | Neochlorogenic acid (NCA) shows a reduction of lipopolysaccharide (LPS)-induced NO production by suppressing iNOS and COX-2 protein expression and production of pro-inflammatory cytokines, such as TNF-α and IL-1β, in BV2 microglia cells. In addition, phosphorylated p38 MAPK and NF-κB p65 are also inhibited by Neochlorogenic acid in activated microglia. iNOS and COX-2 levels are increased in LPS-induced BV2 cells, but this increase is significantly inhibited after treatment with 50 and 100 μM Neochlorogenic acid[1]. |
| Cell Assay | Mouse BV2 microglial cells are maintained in DMEM, supplemented with 5 % FBS and 1 % antibiotic–antimycotic in a humidified incubator with 5 % CO2 at 37°C. Neochlorogenic acid and Dexamethasone as positive control are dissolved in DMSO to a final concentration of 10 mM for the stock solution. Treatments with LPS and/or Neochlorogenic acid are carried out under serum-free conditions. Effects of Neochlorogenic acid are measured on cell viability in lipopolysaccharide (LPS)-stimulated BV2 microglial cells. The cells are treated with or without LPS (4 μg/ml) and Neochlorogenic acid (10, 50, and 100 μM) for 24 h. Dexamethasone (10 μM) is used for positive control. Cell viability is confirmed by the MTT assay. The medium was removed from the wells, MTT was added, and the samples were then incubated for 3 h at 37°C. The formazan crystals were dissolved by adding DMSO, and the absorbance values were measured at 540 nm using a microplate reader[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 665.0±55.0 °C at 760 mmHg |
| Molecular Formula | C16H18O9 |
| Molecular Weight | 354.309 |
| Flash Point | 245.5±25.0 °C |
| Exact Mass | 354.095093 |
| PSA | 164.75000 |
| LogP | -0.36 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.690 |
| Storage condition | 2-8°C |
| Hazard Codes | Xn |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 29182900 |