85650-52-8
| Name | Mirtazapine |
|---|---|
| Synonyms |
Pyrazino[2,1-a]pyrido[2,3-c][2]benzazepine, 1,2,3,4,10,14b-hexahydro-2-methyl-
Zispin (14bR)-2-methyl-1,2,3,4,10,14b-hexahydropyrazino[2,1-a]pyrido[2,3-c][2]benzazepine 1,2,3,4,10,14B-HEXAHYDRO-2-METHYLPYRAZINO[2.1-A]PYRIDO[2.3-C][2]BENZAZEPINE Mirtazapin 1,2,3,4,10,14B-HEXAHYDRO-2-METHYLPYRAZINO[2,1-A]PYRIDO[2,3-C][2]BENZAZEPINE Remeron ORG-3770 UNII:A051Q2099Q 2-Methyl-1,2,3,4,10,14b-hexahydropyrazino[2,1-a]pyrido[2,3-c][2]benzazepine MIRTAZEPINE 2-Methyl-1,2,3,4,10,14b-hexahydrobenzo[c]pyrazino[1,2-a]pyrido[3,2-f]azepine Mirtazanine Avanza MFCD00865427 MIRTAZAPINE ANHYDRUOS EINECS 288-060-6 |
| Description | Mirtazapine is a potent tetracyclic antidepressant.Target: 5-HT ReceptorMirtazapine, the novel antidepressant, has a dual mode of action. It is a noradrenergic and specific serotonergic antidepressant (NaSSA) that acts by antagonizing the adrenergic alpha2-autoreceptors and alpha2-heteroreceptors as well as by blocking 5-HT2 and 5-HT3 receptors [1].Mirtazapine appears to have a substantial ameliorating effect on hot flushes and perspiration bouts. It is postulated that the 5-HT(2A) blocking properties of mirtazapine is accounted in the symptomatic relief of hot flushes [2]. After 4-24 weeks of treatment, mirtazapine induced downregulation of platelet alpha(2A)-adrenoceptors (up to 34%) and Galphai proteins (up to 28%), and the upregulation of GRK 2 (up to 30%). Treatment with mirtazapine reversed this abnormality and induced downregulation of alpha(2A)-adrenoceptor/Galphai complex [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 432.4±45.0 °C at 760 mmHg |
| Melting Point | 114-116ºC |
| Molecular Formula | C17H19N3 |
| Molecular Weight | 265.353 |
| Flash Point | 215.3±28.7 °C |
| Exact Mass | 265.157898 |
| PSA | 19.37000 |
| LogP | 2.75 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: ~8 mg/mL, soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H336 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
|
~94% 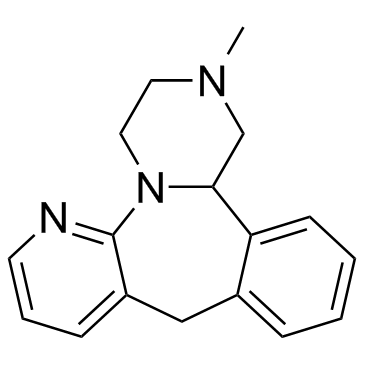
85650-52-8 |
| Literature: Singer, Claude; Liberman, Anita; Finkelstein, Nina Patent: US2001/51718 A1, 2001 ; Title/Abstract Full Text Show Details Singer, Claude; Liberman, Anita; Finkelstein, Nina Patent: US2003/69417 A1, 2003 ; |
|
~82% 
85650-52-8 |
| Literature: N.V. ORGANON Patent: WO2008/125578 A2, 2008 ; Location in patent: Page/Page column 16 ; |
|
~55% 
85650-52-8 |
| Literature: N.V. Organon Patent: US2008/255349 A1, 2008 ; Location in patent: Page/Page column 9 ; |
|
~% 
85650-52-8 |
| Literature: WO2008/125577 A1, ; Page/Page column 22 ; |
|
~% 
85650-52-8 |
| Literature: Organic Preparations and Procedures International, , vol. 39, # 4 p. 399 - 402 |
|
~% 
85650-52-8 |
| Literature: Organic Preparations and Procedures International, , vol. 39, # 4 p. 399 - 402 |
|
~% 
85650-52-8 |
| Literature: Organic Preparations and Procedures International, , vol. 39, # 4 p. 399 - 402 |
|
~% 
85650-52-8 |
| Literature: Organic Preparations and Procedures International, , vol. 39, # 4 p. 399 - 402 |
| Precursor 7 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |





![()-1,2,3,4,10,14b-hexahydro-2-methylpyrazino[2,1-a]pyrido[2,3-c][2]benzazepine maleate (1:1) structure](https://image.chemsrc.com/caspic/075/85650-53-9.png)
