976-71-6
| Name | Canrenone |
|---|---|
| Synonyms |
Phanurane
Luvion ALDADIENE (17a)-17-Hydroxy-3-oxopregna-4,6-diene-21-carboxylic acid g-lactone 17a-(2-Carboxyethyl)-17b-hydroxyandrosta-4,6-dien-3-one Lactone Canrenone Spiro[17H-cyclopenta[a]phenanthrene-17,2'(5'H)-furan]-3,5'(2H)-dione, 1,3',4',8,9,10,11,12,13,14,15,16-dodecahydro-10,13-dimethyl-, (8R,9S,10R,13S,14S,17R)- 17a-(2-Carboxyethyl)-17b-hydroxy-3-oxoandrosta-4,6-diene Lactone 3-(3-Oxo-17b-hydroxy-4,6-androstadien-17a-yl)propionic Acid g-Lactone Contaren 6-Dehydrotestosterone-17a-propionic Acid g-Lactone CANRENONE-D4 (8R,9S,10R,13S,14S,17R)-10,13-Dimethyl-1,8,9,10,11,12,13,14,15,16-decahydro-3'H-spiro[cyclopenta[a]phenanthrene-17,2'-furan]-3,5'(2H,4'H)-dione 11614 R.P. |
| Description | Canrenone (Aldadiene; SC9376; SC14266) is an aldosterone antagonist extensively used as a diuretic agent. |
|---|---|
| Related Catalog | |
| Target |
Target: Aldosterone[1] |
| In Vitro | Canrenone inhibits the production of eortieosterone, 18-hydroxydesoxyeortieosterone, 18-hydroxycorticosterone and aldosterone in a dose-dependent manner[1]. Canrenone dose-dependently reduces platelet-derived growth factor–induced cell proliferation and motility. Canrenone inhibits the activity of the Na+/H+ exchanger 1 induced by platelet-derived growth factor[2]. |
| In Vivo | Canrenone is the principal active metabolite of Spironolactone in the rat only for a limited period. During chronic treatment a difference developed between the effect of Spironolactone and Canrenone on the RAAS indicating a decrease in the anti-mineralocorticoid activity of Canrenone and an increase in the efficacy of Spironolactone[3]. |
| Cell Assay | Confluent Hepatic Stellate Cells (HSC) are incubated in SFIF medium for 24 hours and exposed to increasing concentrations of canrenone (1, 5, 10, 25 μM). Cell viability is evaluated by the trypan blue dye exclusion test at the end of a 24- to 48-hour incubation period[2]. |
| Animal Admin | Rats[3] Canrenone (CAN) is given orally in two different doses (10.25, 20.5 mg/mL) to Male SPF Sprague-Dawley rats for 6 weeks. To determine the Na+, K+, fluid and aldosterone excretion the urine of the rats destined to be killed after 6 weeks is collected at weekly intervals[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 541.1±50.0 °C at 760 mmHg |
| Melting Point | 158-160ºC |
| Molecular Formula | C22H28O3 |
| Molecular Weight | 340.456 |
| Flash Point | 237.6±30.2 °C |
| Exact Mass | 340.203857 |
| PSA | 43.37000 |
| LogP | 2.99 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.581 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS08, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351-H411 |
| Precautionary Statements | P280 |
| Hazard Codes | Xn,N |
| Risk Phrases | 40-51/53 |
| Safety Phrases | 36/37-61 |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 29329990 |
| Precursor 7 | |
|---|---|
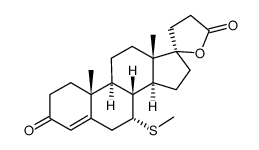
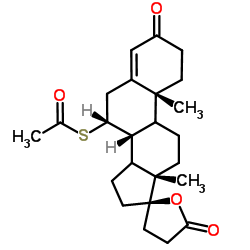
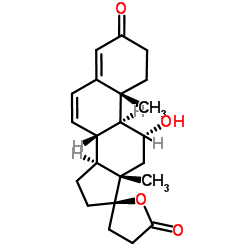
| DownStream 6 | |




![5'-hydroxyspiro[pregnane-17,2'-tetrahydrofuran]-5-en-3-one structure](https://image.chemsrc.com/caspic/151/121936-43-4.png)

![(1R,8S,9S,10R,11R,13S,14S,17R)-1,11-dihydroxy-10,13-dimethyl-1,3',4',8,9,10,11,12,13,14,15,16-dodecahydro-5'H-spiro[cyclopenta[a]phenanthrene-17,2'-furan]-3,5'(2H)-dione structure](https://image.chemsrc.com/caspic/458/1363502-03-7.png)
