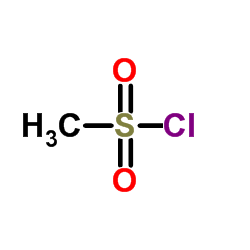
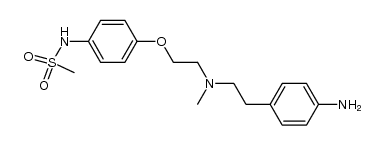
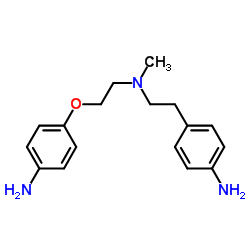
115256-11-6
| Name | dofetilide |
|---|---|
| Synonyms |
N-[4-[2-[Methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]methanesulfon Amide
Dofetilidum Xelide N-[4-[2-[2-[4-(methanesulfonamido)phenoxy]ethyl-methylamino]ethyl]phenyl]methanesulfonamide MFCD00869707 Dofetilide 1-MSPMPE b-((p-Methanesulfonamidophenethyl)methylamino)methanesulfono-p-phenetidide N-(4-{2-[Methyl(2-{4-[(methylsulfonyl)amino]phenoxy}ethyl)amino]ethyl}phenyl)methanesulfonamide Dofetilida Methanesulfonamide, N-[4-[2-[methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]- Tikosyn |
| Description | Dofetilide(Tikosyn) is a class III antiarrhythmic agent.Target: Potassium ChannelIn patients with congestive heart failure and reduced left ventricular function, dofetilide was effective in converting atrial fibrillation, preventing its recurrence, and reducing the risk of hospitalization for worsening heart failure. Dofetilide had no effect on mortality [1]. dofetilide preferentially blocks open (or activated) channels and that the fast inactivation may competitively slow the binding kinetics. Dofetilide acts as a slow-onset/slow-offset open channel blocker of this current at nanomolar concentrations [2]. |
|---|---|
| Related Catalog | |
| References |
[1]. Maisch, B., et al., [Pregnancy and cardiomyopathies]. Herz, 2003. 28(3): p. 196-208. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 614.1±65.0 °C at 760 mmHg |
| Melting Point | 147-1490C |
| Molecular Formula | C19H27N3O5S2 |
| Molecular Weight | 441.565 |
| Flash Point | 325.2±34.3 °C |
| Exact Mass | 441.139221 |
| PSA | 121.57000 |
| LogP | 1.56 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.614 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H360 |
| Precautionary Statements | P201-P308 + P313 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
|
~92% 
115256-11-6 |
| Literature: Organic Letters, , vol. 13, # 10 p. 2564 - 2567 |
|
~90% 
115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
|
~% 
115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~% 
115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~% 
115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~52% 
115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~% 
115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
|
~% 
115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
|
~% 
115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |