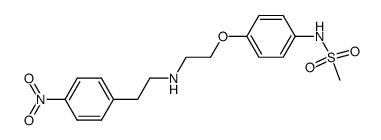
Dofetilide

Dofetilide structure
|
Common Name | Dofetilide | ||
|---|---|---|---|---|
| CAS Number | 115256-11-6 | Molecular Weight | 441.565 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 614.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C19H27N3O5S2 | Melting Point | 147-1490C | |
| MSDS | Chinese USA | Flash Point | 325.2±34.3 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of DofetilideDofetilide(Tikosyn) is a class III antiarrhythmic agent.Target: Potassium ChannelIn patients with congestive heart failure and reduced left ventricular function, dofetilide was effective in converting atrial fibrillation, preventing its recurrence, and reducing the risk of hospitalization for worsening heart failure. Dofetilide had no effect on mortality [1]. dofetilide preferentially blocks open (or activated) channels and that the fast inactivation may competitively slow the binding kinetics. Dofetilide acts as a slow-onset/slow-offset open channel blocker of this current at nanomolar concentrations [2]. |
| Name | dofetilide |
|---|---|
| Synonym | More Synonyms |
| Description | Dofetilide(Tikosyn) is a class III antiarrhythmic agent.Target: Potassium ChannelIn patients with congestive heart failure and reduced left ventricular function, dofetilide was effective in converting atrial fibrillation, preventing its recurrence, and reducing the risk of hospitalization for worsening heart failure. Dofetilide had no effect on mortality [1]. dofetilide preferentially blocks open (or activated) channels and that the fast inactivation may competitively slow the binding kinetics. Dofetilide acts as a slow-onset/slow-offset open channel blocker of this current at nanomolar concentrations [2]. |
|---|---|
| Related Catalog | |
| References |
[1]. Maisch, B., et al., [Pregnancy and cardiomyopathies]. Herz, 2003. 28(3): p. 196-208. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 614.1±65.0 °C at 760 mmHg |
| Melting Point | 147-1490C |
| Molecular Formula | C19H27N3O5S2 |
| Molecular Weight | 441.565 |
| Flash Point | 325.2±34.3 °C |
| Exact Mass | 441.139221 |
| PSA | 121.57000 |
| LogP | 1.56 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.614 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H360 |
| Precautionary Statements | P201-P308 + P313 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
|
~92% 
Dofetilide CAS#:115256-11-6 |
| Literature: Organic Letters, , vol. 13, # 10 p. 2564 - 2567 |
|
~90% 
Dofetilide CAS#:115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
|
~% 
Dofetilide CAS#:115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~% 
Dofetilide CAS#:115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~% 
Dofetilide CAS#:115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~52% 
Dofetilide CAS#:115256-11-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1151 - 1155 |
|
~% 
Dofetilide CAS#:115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
|
~% 
Dofetilide CAS#:115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
|
~% 
Dofetilide CAS#:115256-11-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 19 p. 2153 - 2157 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
|
Synchronous systolic subcellular Ca2+-elevations underlie ventricular arrhythmia in drug-induced long QT type 2.
Circ. Arrhythm. Electrophysiol. 8 , 703-12, (2015) Repolarization delay is a common clinical problem, which can promote ventricular arrhythmias. In myocytes, abnormal sarcoplasmic reticulum Ca(2+)-release is proposed as the mechanism that causes early... |
|
|
The Link between Inactivation and High-Affinity Block of hERG1 Channels.
Mol. Pharmacol. 87 , 1042-50, (2015) Block of human ether-à-go-go-related gene 1 (hERG1) K(+) channels by many drugs delays cardiac repolarization, prolongs QT interval, and is associated with an increased risk of cardiac arrhythmia. Pre... |
|
|
Relevance of calmodulin/CaMKII activation for arrhythmogenesis in the AV block dog.
Heart Rhythm 9(11) , 1875-83, (2012) The calcium-dependent signaling molecules calcineurin and calcium/calmodulin-dependent protein kinase II (CaMKII) both have been linked to decompensated hypertrophy and arrhythmias. CaMKII is also bel... |
| N-[4-[2-[Methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]methanesulfon Amide |
| Dofetilidum |
| Xelide |
| N-[4-[2-[2-[4-(methanesulfonamido)phenoxy]ethyl-methylamino]ethyl]phenyl]methanesulfonamide |
| MFCD00869707 |
| Dofetilide |
| 1-MSPMPE |
| b-((p-Methanesulfonamidophenethyl)methylamino)methanesulfono-p-phenetidide |
| N-(4-{2-[Methyl(2-{4-[(methylsulfonyl)amino]phenoxy}ethyl)amino]ethyl}phenyl)methanesulfonamide |
| Dofetilida |
| Methanesulfonamide, N-[4-[2-[methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]- |
| Tikosyn |


![1-(4-methanesulphonamidophenoxy)-2-[N-methyl-N-(4-aminophenethyl)amino]ethane structure](https://image.chemsrc.com/caspic/174/115256-12-7.png)

![N-methyl-2-(4-nitrophenoxy)-N-[2-(4-nitrophenyl)ethyl]ethanamine structure](https://image.chemsrc.com/caspic/249/115287-37-1.png)
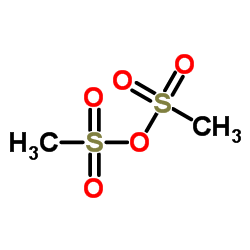
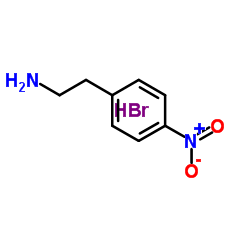
![1-(4-methanesulphonamidophenoxy)-2-[N-methyl-N-(4-nitrophenethyl)amino]ethane structure](https://image.chemsrc.com/caspic/467/115256-44-5.png)