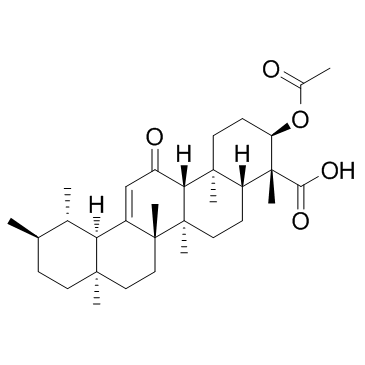
67416-61-9
| Name | Acetyl-11-keto-b-boswellic acid |
|---|---|
| Synonyms |
3-O-Acetyl-11-keto-β-boswellic acid
Urs-12-en-24-oic acid, 3-(acetyloxy)-11-oxo-, (3α)- (3α)-3-Acetoxy-11-oxours-12-en-24-oic acid (3α)-3-(acetyloxy)-11-oxours-12-en-24-oic acid 3-Acetyl-11-keto-β-boswellic Acid AKBA |
| Description | Acetyl-11-Keto-β-Boswellic Acid (AKBA) is an active triterpenoid compound from the extract of Boswellia serrate; a novel Nrf2 activator.IC50 value:Target: Nrf2 activatorin vitro: AKBA significantly reduced infarct volumes and apoptotic cells, and also increased neurologic scores by elevating the Nrf2 and HO-1 expression in brain tissues in middle cerebral artery occlusion (MCAO) rats at 48 hours post reperfusion. In primary cultured neurons, AKBA increased the Nrf2 and HO-1 expression, which provided protection against OGD-induced oxidative insult. Additionally, AKBA treatment increased Nrf2 binding activity to antioxidant-response elements (ARE) [1]. AKBA significantly inhibited human colon adenocarcinoma growth, showing arrest of the cell cycle in G1-phase and induction of apoptosis[3]. AKBA triggered significant lipolysis in 3T3-L1 adipocytes as shown by reduced neutral lipids in cytosol and increased free fatty acids in culture medium. Increased lipolysis by AKBA was accompanied by up-regulation of lipolytic enzymes, adipocyte triglyceride lipase (ATGL) and hormone sensitive lipase (HSL), and a decreased expression of lipid droplet stability regulator perilipin. In addition, AKBA treatment reduced phenotypic markers of mature adipocyte aP2, adiponectin and glut-4 in mature adipocytes [5].in vivo: AKBA significantly prevented the formation of intestinal adenomatous polyps without toxicity to mice. AKBA's activity both in the prevention of small intestinal and colonic polyps was more potently than aspirin. Histopathologic examination revealed that AKBA's effect, that is the reduction of polyp size and degree of dysplasia, was more prominent in larger sized polyps, especially those originating in colon [2]. AKBA administration in mice effectively delayed the growth of HT-29 xenografts without signs of toxicity. The activity of AKBA was more potent than that of aspirin [3]. AKBA exhibited anti-cancer activity in vitro and in vivo. With oral application in mice, AKBA significantly inhibited SGC-7901 and MKN-45 xenografts without toxicity [4]. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 600.3±55.0 °C at 760 mmHg |
| Molecular Formula | C32H48O5 |
| Molecular Weight | 512.721 |
| Flash Point | 184.4±25.0 °C |
| Exact Mass | 512.350159 |
| PSA | 80.67000 |
| LogP | 8.00 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.549 |
| Storage condition | −20°C |
|
~%
Detail
|
| Literature: WO2006/95355 A1, ; Page/Page column 11-12 ; |
| Precursor 4 | |
|---|---|
| DownStream 1 | |