CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
MA1050000
-
CHEMICAL NAME :
-
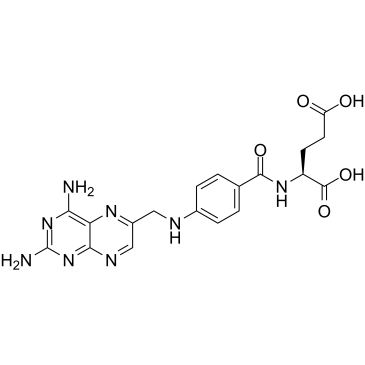
Glutamic acid, N-(p-(((2,4-diamino-6-pteridinyl)methyl)amino)benzoyl )-, L-
-
CAS REGISTRY NUMBER :
-
54-62-6
-
BEILSTEIN REFERENCE NO. :
-
0069045
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
56
-
MOLECULAR FORMULA :
-
C19-H20-N8-O5
-
MOLECULAR WEIGHT :
-
440.47
-
WISWESSER LINE NOTATION :
-
T66 BN DN GN JNJ CZ EZ H1MR DVMYVQ2VQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
120 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - anorexia (human) Gastrointestinal - other changes Skin and Appendages - hair
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2500 ug/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1900 ug/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - alteration in gastric secretion Gastrointestinal - hypermotility, diarrhea Blood - changes in bone marrow (not otherwise specified)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
246 ug/kg/5D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Blood - changes in other cell count (unspecified) Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
24300 ug/kg/9D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Blood - changes in bone marrow (not otherwise specified) Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
22200 ug/kg/20D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Blood - changes in bone marrow (not otherwise specified) Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1200 ug/kg/17W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - leukemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
240 ug/kg
-
SEX/DURATION :
-
female 55-66 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
580 ug/kg
-
SEX/DURATION :
-
female 6-8 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2880 ug/kg
-
SEX/DURATION :
-
female 56-67 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - Apgar score (human only)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
165 ug/kg
-
SEX/DURATION :
-
female 40-44 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
188 ug/kg
-
SEX/DURATION :
-
female 54-55 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
151 ug/kg
-
SEX/DURATION :
-
female 35-36 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
240 ug/kg
-
SEX/DURATION :
-
female 1-30 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
240 ug/kg
-
SEX/DURATION :
-
female 53-55 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
15 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
150 ug/kg
-
SEX/DURATION :
-
female 9-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
112 ug/kg
-
SEX/DURATION :
-
female 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
75 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
150 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - homeostasis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
150 ug/kg
-
SEX/DURATION :
-
female 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
280 ug/kg
-
SEX/DURATION :
-
male 28 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
75 ug/kg
-
SEX/DURATION :
-
female 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
75 ug/kg
-
SEX/DURATION :
-
female 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - body wall
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
50 ug/kg
-
SEX/DURATION :
-
female 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
3 mg/kg
-
SEX/DURATION :
-
female 1 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2500 ug/kg
-
SEX/DURATION :
-
female 18-27 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 18-27 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 16-25 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 ug/kg
-
SEX/DURATION :
-
female 20-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
15 mg/kg
-
SEX/DURATION :
-
female 15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1100 ug/kg
-
SEX/DURATION :
-
female 12-33 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
Micronucleus test
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
2 mg/kg/5D
-
REFERENCE :
-
PNASA6 Proceedings of the National Academy of Sciences of the United States of America. (National Academy of Sciences, Printing & Pub. Office, 2101 Constitution Ave., Washington, DC 20418) V.1- 1915- Volume(issue)/page/year: 72,4425,1975 *** REVIEWS *** TOXICOLOGY REVIEW BCSTB5 Biochemical Society Transactions. (Biochemical Soc. Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1973- Volume(issue)/page/year: 2,695,1974 TOXICOLOGY REVIEW PLMJAP Pahlavi Medical Journal. (Shiraz, Iran) V.1-9, 1970-78. Volume(issue)/page/year: 6,160,1975 TOXICOLOGY REVIEW JAMAAP JAMA, Journal of the American Medical Association. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1883- Volume(issue)/page/year: 172,1765,1960 TOXICOLOGY REVIEW 32XPAD "Teratology," Berry, C.L., and D.E. Poswillo, eds., New York, Springer, 1975 Volume(issue)/page/year: -,49,1975 TOXICOLOGY REVIEW JOGBAS Journal of Obstetrics and Gynaecology of the British Commonwealth. (London, UK) V.68-81, 1961-74. For publisher information, see BJOGAS. Volume(issue)/page/year: 75,307,1968 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW ADVPA3 Advances in Pharmacology. (New York, NY) V.1-6, 1962-68. For publisher information, see AVPCAQ. Volume(issue)/page/year: 4,263,1966 TOXICOLOGY REVIEW ATXKA8 Archiv fuer Toxikologie. (Berlin, Fed. Rep. Ger.) V.15-31, 1954-74. For publisher information, see ARTODN. Volume(issue)/page/year: 28,135,1971
|





![4-(N-[2,4-DIAMINO-6-PTERIDINYLMETHYL]-AMINO)BENZOIC ACID SODIUM SALT structure](https://image.chemsrc.com/caspic/389/36093-85-3.png)
![4-[[2-(2,4-diaminopteridin-6-yl)acetyl]amino]benzoic acid structure](https://image.chemsrc.com/caspic/168/89043-75-4.png)
![dimethyl 2-[[4-[(2,4-diaminopteridin-6-yl)methyl-(trideuteriomethyl)amino]benzoyl]amino]pentanedioate structure](https://image.chemsrc.com/caspic/178/432545-60-3.png)
