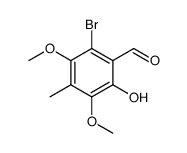
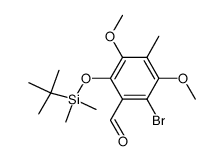
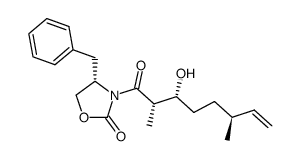
183202-73-5
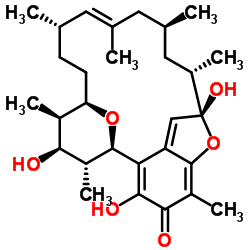
| Name | Kendomycin |
|---|---|
| Synonyms |
(1R,9S,10S,12S,14E,16S,19R,20R,21S,22R)-3,9,21-Trihydroxy-5,10,12,14,16,20,22-heptamethyl-23,24-dioxatetracyclo[17.3.1.1.0]tetracosa-2,5,7,14-tetraen-4-one
kendomycin from streptomyces violaceoruber Kendomycin 1,19:5,9-Diepoxybenzocyclooctadecen-3(5H)-one, 6,7,8,9,10,11,12,15,16,17,18,19-dodecahydro-4,7,19-trihydroxy-2,6,8,12,14,16,18-heptamethyl-, (5R,6R,7S,8R,9R,12S,13E,16S,18S,19S)- |
| Description | Kendomycin ((−)-TAN 2162) is a polyketide antibiotic with remarkable antibacterial and cancer cells cytotoxic activities. Kendomycin tends to be bacteriostatic rather than bactericidal and inhibits the growth of the methicillin-resistant Staphylococcus aureus (MRSA) strain COL at a low concentration (MIC of 5 μg/mL). Kendomycin is a potent antagonist of the endothelin receptor and a calcitonin receptor agonist which plays its role as an anti-osteoporotic agent[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 678.5±55.0 °C at 760 mmHg |
| Molecular Formula | C29H42O6 |
| Molecular Weight | 486.640 |
| Flash Point | 219.9±25.0 °C |
| Exact Mass | 486.298126 |
| PSA | 96.22000 |
| LogP | 5.94 |
| Vapour Pressure | 0.0±4.7 mmHg at 25°C |
| Index of Refraction | 1.576 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 10 | |
|---|---|
| DownStream 0 | |