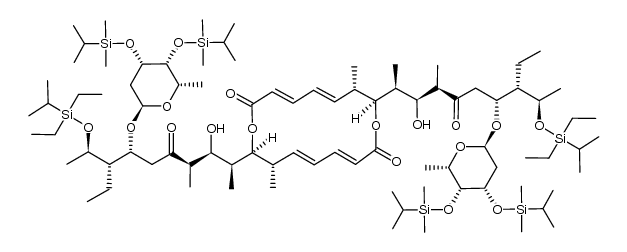
37318-06-2
| Name | elaiophylin |
|---|---|
| Synonyms |
(3E,5E,7S,8S,11E,13E,15S,16S)-8,16-Bis{(2S,3R,4S)-4-[(2R,4R,5R,6R)-4-{[(2R,4S,5S,6S)-4,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-5-ethyl-2-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]-3-hydroxypentan-2-yl}-7,15-dimethyl-1,9-dioxacyclohexadeca-3,5,11,13-tetraene-2,10-dione (non-preferred name)
(3E,5E,7S,8S,11E,13E,15S,16S)-8,16-Bis{(2S,3R,4S)-4-[(2R,4R,5R,6R)-4-{[(2R,4S,5S,6S)-4,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-5-ethyl-2-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]-3-hydroxy-2-pentanyl}-7,15-dimethyl-1,9-dioxacyclohexadeca-3,5,11,13-tetraene-2,10-dione 5001B gopalamicin Azalomycin Azalomycin-B,Gopalamicin,SNA 4606-3 Antibiotic 56-62 Elaiofilin Salbomycin Elaiophylin,Salbomycin,Gopalamicin,SNA 4606-3,Azalomycin-B (3E,5E,7S,8S,11E,13E,15S,16S)-8,16-bis[(2S,3R,4S)-4-[(2R,4R,5R,6R)-4-[(2R,4S,5S,6S)-4,5-dihydroxy-6-methyloxan-2-yl]oxy-5-ethyl-2-hydroxy-6-methyloxan-2-yl]-3-hydroxypentan-2-yl]-7,15-dimethyl-1,9-dioxacyclohexadeca-3,5,11,13-tetraene-2,10-dione Antibiotic 5001B azalomycin B Elaiophylin |
| Description | Elaiophylin (Azalomycin B; Gopalamicin; Efomycin E) is an autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells[1]. |
|---|---|
| Related Catalog | |
| Target |
Autophagy[1] |
| In Vitro | Elaiophylin-mediated autophagy inhibition and lysosomal dysfunction affect ovarian cancer cell survival during hypoxia. Exposure to Elaiophylin (0.025-0.5 μM; 24 hours) causes a significant increase in ovarian cancer SKOV3 cell death in hypoxia conditions[1]. In both the SKOV3 and A2780 cell lines, Elaiophylin (0.25, 0.5, 0.75 μM; 24 hours) treatment leads to significant activation of cleaved CASP9/caspase-9 and PARP1 and downregulation of BIRC5/survivin in a concentration-dependent manner[1]. Cell Viability Assay[1] Cell Line: Ovarian cancer SKOV3 cells. Concentration: 0.025, 0.05, 0.1, 0.2, 0.5 μM Incubation Time: 24 hours Result: Caused a significant increase in ovarian cancer SKOV3 cells death in hypoxia conditions. Western Blot Analysis[1] Cell Line: Ovarian cancer SKOV3 cells; A2780 cells Concentration: 0.25, 0.5, 0.75 μM Incubation Time: 24 hours Result: Treatment led to significant activation of cleaved CASP9/caspase-9 and PARP1 and downregulation of BIRC5/survivin in a concentration-dependent manner. |
| In Vivo | Treatment with 2 mg/kg Elaiophylin (given i.p. every 2 days for 21 days; in BALB/C athymic mice) significantly suppresses ovarian cancer SKOV3 cells growth compared with DMSO treatment, resulting in a 72% decrease in the average daily tumor growth rate compared with DMSO treatment [1]. Lower doses of Elaiophylin as a single agent exert significant antitumor activity, while higher doses lead to intestinal toxicity. Administration of a lower dose (2 mg/kg) of Elaiophylin as a single agent achieves a significant antitumor effect without toxicity in an orthotopic ovarian cancer model with metastasis. Toxic reactions are observed only in the 8 mg/kg group[1]. Animal Model: 4-wk-old BALB/C athymic mice with ovarian cancer SKOV3 cells[1] Dosage: 1 or 2 mg/kg Administration: Given i.p. every 2 days for 21 days Result: Treatment with 2 mg/kg significantly suppressed ovarian cancer SKOV3 cells growth compared with DMSO treatment. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 1080.5±65.0 °C at 760 mmHg |
| Molecular Formula | C54H88O18 |
| Molecular Weight | 1025.27 |
| Flash Point | 295.8±27.8 °C |
| Exact Mass | 1024.597046 |
| PSA | 269.82000 |
| LogP | 2.59 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.569 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~21% 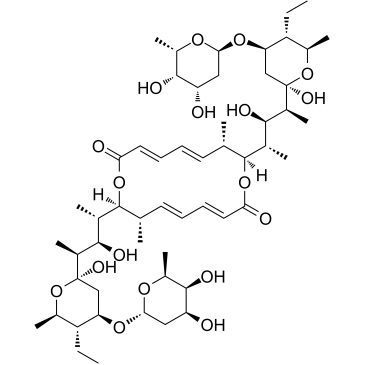
37318-06-2 |
| Literature: Toshima; Tatsuta; Kinoshita Tetrahedron Letters, 1986 , vol. 27, # 39 p. 4741 - 4744 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |