Phenanthridin-6(5H)-one

Phenanthridin-6(5H)-one structure
|
Common Name | Phenanthridin-6(5H)-one | ||
|---|---|---|---|---|
| CAS Number | 1015-89-0 | Molecular Weight | 195.22 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 274.8±7.0 °C at 760 mmHg | |
| Molecular Formula | C13H9NO | Melting Point | 290-292 °C(lit.) | |
| MSDS | USA | Flash Point | 158.7±3.1 °C | |
Use of Phenanthridin-6(5H)-one6(5H)-Phenanthridinone is a potent PARP-1 inhibitor and immunomodulator. 6(5H)-Phenanthridinone inhibits cell proliferation and can be used in cancer research[1]. |
| Name | phenanthridone |
|---|---|
| Synonym | More Synonyms |
| Description | 6(5H)-Phenanthridinone is a potent PARP-1 inhibitor and immunomodulator. 6(5H)-Phenanthridinone inhibits cell proliferation and can be used in cancer research[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 274.8±7.0 °C at 760 mmHg |
| Melting Point | 290-292 °C(lit.) |
| Molecular Formula | C13H9NO |
| Molecular Weight | 195.22 |
| Flash Point | 158.7±3.1 °C |
| Exact Mass | 195.068420 |
| PSA | 32.86000 |
| LogP | 2.81 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.642 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SG0370000 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Molecular design, synthesis and docking study of benz[b]oxepines and 12-oxobenzo[c]phenanthridinones as topoisomerase 1 inhibitors.
Bioorg. Med. Chem. Lett. 19 , 2444-7, (2009) Benz[b]oxepines 4a-g and 12-oxobenzo[c]phenanthridines 5a-d were designed and synthesized as constrained forms of 3-arylisoquinolines through an intramolecular radical cyclization reaction. Radical cy... |
|
|
Novel isoquinolinone-derived inhibitors of poly(ADP-ribose) polymerase-1: pharmacological characterization and neuroprotective effects in an in vitro model of cerebral ischemia.
J. Pharmacol. Exp. Ther. 305(3) , 943-9, (2003) Excessive activation of poly(ADP-ribose) polymerase-1 (PARP-1), a nuclear enzyme catalyzing the transfer of ADP-ribose units from NAD to acceptor proteins, induces cellular energy failure by NAD and A... |
|
|
Total synthesis of 7-deoxypancratistatin-1-carboxaldehyde and carboxylic acid via solvent-free intramolecular aziridine opening: phenanthrene to phenanthridone cyclization strategy.
Org. Lett. 10(3) , 361-4, (2008) Solid-state silica-gel-catalyzed opening of aziridine 6 provided phenanthrene 7, whose oxidative cleavage, recyclization, and further elaboration furnished the C-1 aldehyde and carboxylic acid derivat... |
| phenanthridone |
| 6-phenanthridinol |
| phenanthridin-6(5H)-one |
| EINECS 213-804-3 |
| phenanthridin-6-ol |
| 6-(5H)-Phenanthridinone |
| MFCD00004988 |
| 6-Hydroxyphenanthridine |
| 5,6-dihydrophenanthridin-6-one |
| 6(5H)-Phenanthridinone |
| 6(5H)-Phenanthridone |
| 5H-phenanthridin-6-one |
 CAS#:201230-82-2
CAS#:201230-82-2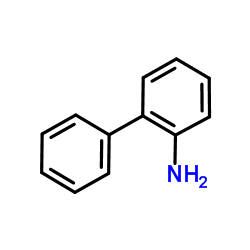 CAS#:90-41-5
CAS#:90-41-5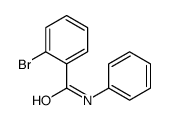 CAS#:10282-57-2
CAS#:10282-57-2![[1,1'-Biphenyl]-2-carboxamide Structure](https://image.chemsrc.com/caspic/075/13234-79-2.png) CAS#:13234-79-2
CAS#:13234-79-2 CAS#:54147-90-9
CAS#:54147-90-9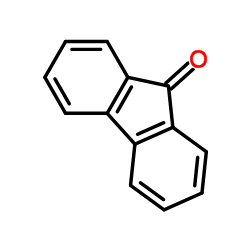 CAS#:486-25-9
CAS#:486-25-9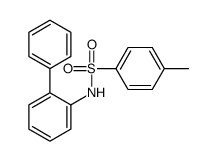 CAS#:24310-30-3
CAS#:24310-30-3 CAS#:2157-52-0
CAS#:2157-52-0 CAS#:609-73-4
CAS#:609-73-4 CAS#:610-97-9
CAS#:610-97-9 CAS#:229-87-8
CAS#:229-87-8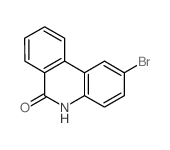 CAS#:27353-48-6
CAS#:27353-48-6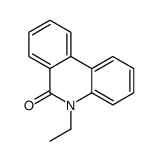 CAS#:25491-54-7
CAS#:25491-54-7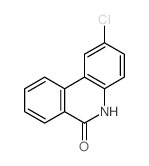 CAS#:27353-44-2
CAS#:27353-44-2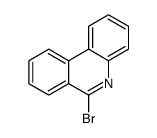 CAS#:17613-40-0
CAS#:17613-40-0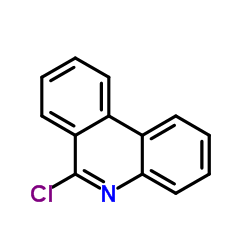 CAS#:15679-03-5
CAS#:15679-03-5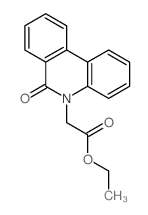 CAS#:37045-19-5
CAS#:37045-19-5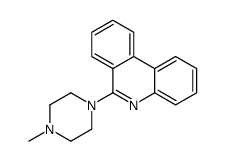 CAS#:23441-13-6
CAS#:23441-13-6 CAS#:137531-20-5
CAS#:137531-20-5 CAS#:27799-79-7
CAS#:27799-79-7
