Endoxifen (Z-isomer hydrochloride)
Modify Date: 2024-01-05 13:44:53
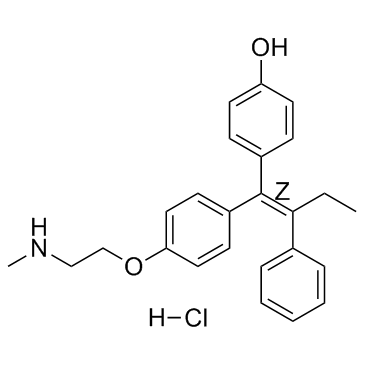
Endoxifen (Z-isomer hydrochloride) structure
|
Common Name | Endoxifen (Z-isomer hydrochloride) | ||
|---|---|---|---|---|
| CAS Number | 1032008-74-4 | Molecular Weight | 409.948 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H28ClNO2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Endoxifen (Z-isomer hydrochloride)Endoxifen Z-isomer hydrochloride is the most important Tamoxifen metabolite responsible for eliciting the anti-estrogenic effects of this drug in breast cancer cells expressing estrogen receptor-alpha (ERα). Endoxifen inhibits hERG tail currents at 50 mV in a concentration-dependent manner with IC50 values of 1.6 μM.IC50 value: 1.6 μM [1]Target: hERG Potassium Channel, Estrogen Receptor/ERREndoxifen is considered a prodrug, since it has a much higher potency for the estrogen receptor than its parent drug. Endoxifen inhibits the hERG channel protein trafficking to the plasma membrane in a concentration-dependent manner with Endoxifen being more potent than Tamoxifen. [1] Endoxifen is also shown to be a more potent inhibitor of estrogen target genes when ERβ is expressed. Additionally, low concentrations of Endoxifen observed in Tamoxifen treated patients with deficient CYP2D6 activity (20 to 40 nM) markedly inhibit estrogen-induced cell proliferation rates in the presence of ERβ, whereas much higher Endoxifen concentrations are needed when ERβ is absent.[2] |
| Name | 4-[(Z)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenol,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Endoxifen Z-isomer hydrochloride is the most important Tamoxifen metabolite responsible for eliciting the anti-estrogenic effects of this drug in breast cancer cells expressing estrogen receptor-alpha (ERα). Endoxifen inhibits hERG tail currents at 50 mV in a concentration-dependent manner with IC50 values of 1.6 μM.IC50 value: 1.6 μM [1]Target: hERG Potassium Channel, Estrogen Receptor/ERREndoxifen is considered a prodrug, since it has a much higher potency for the estrogen receptor than its parent drug. Endoxifen inhibits the hERG channel protein trafficking to the plasma membrane in a concentration-dependent manner with Endoxifen being more potent than Tamoxifen. [1] Endoxifen is also shown to be a more potent inhibitor of estrogen target genes when ERβ is expressed. Additionally, low concentrations of Endoxifen observed in Tamoxifen treated patients with deficient CYP2D6 activity (20 to 40 nM) markedly inhibit estrogen-induced cell proliferation rates in the presence of ERβ, whereas much higher Endoxifen concentrations are needed when ERβ is absent.[2] |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C25H28ClNO2 |
|---|---|
| Molecular Weight | 409.948 |
| Exact Mass | 409.180847 |
| PSA | 41.49000 |
| LogP | 6.55240 |
| Storage condition | -20℃ |
|
~% 
Endoxifen (Z-is... CAS#:1032008-74-4 |
| Literature: Fauq, Abdul H.; Maharvi, Ghulam M.; Sinha, Dola Bioorganic and Medicinal Chemistry Letters, 2010 , vol. 20, # 10 p. 3036 - 3038 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| UNII-308PA1L567 |
| 4-[(1Z)-1-{4-[2-(Methylamino)ethoxy]phenyl}-2-phenyl-1-buten-1-yl]phenol hydrochloride (1:1) |
| Z-Endoxifen hydrochloride |
| 4-Hydroxy-N-desmethyl-tamoxifen,hydrochloride |
| Phenol, 4-[(1Z)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]-, hydrochloride (1:1) |
| Endoxifen hydrochloride |
| Endoxifen (Z-isomer hydrochloride) |