ferulic acid
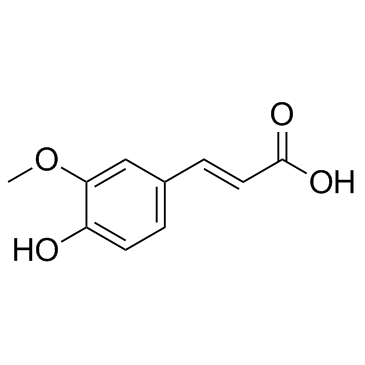
ferulic acid structure
|
Common Name | ferulic acid | ||
|---|---|---|---|---|
| CAS Number | 1135-24-6 | Molecular Weight | 194.184 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 372.3±27.0 °C at 760 mmHg | |
| Molecular Formula | C10H10O4 | Melting Point | 168-172 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 150.5±17.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of ferulic acidFerulic acid is a novel fibroblast growth factor receptor 1 (FGFR1) inhibitor with IC50s of 3.78 and 12.5 μM for FGFR1 and FGFR2, respectively. |
| Name | ferulic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Ferulic acid is a novel fibroblast growth factor receptor 1 (FGFR1) inhibitor with IC50s of 3.78 and 12.5 μM for FGFR1 and FGFR2, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3.78 μM (FGFR1), 12.5 μM (FGFR2)[1] |
| In Vitro | Ferulic acid (FA) is a novel fibroblast growth factor receptor 1 (FGFR1) inhibitor with IC50s of 3.78 and 12.5 μM for FGFR1 and FGFR2, respectively. Ferulic acid exhibits great inhibitory activity on FGFR1 with an inhibitory rate of 92% at 1 µM. The proliferation of HUVEC stimulated by FGF1 is markedly decreased after Ferulic acid treatment ranging from 5 to 40 μM for 24 h. Ferulic acid does not exert significant cell viability up to 20 μM, but over 30 μM Ferulic acid exhibits a cytotoxic effect in HUVEC compare to the control. Ferulic acid inhibits FGF1-induced HUVEC migration and invasion in a dose-dependent manner. Ferulic acid markedly suppresses the FGF1-induced phosphorylation of PI3K and Akt. Ferulic acid treatments significantly inhibit MMP-2 and MMP-9 expression stimulated by FGF1[1]. |
| In Vivo | Treatment with Ferulic acid (FA) potently inhibits FGF1-induced neovascularization. It is found that intragastric administration of Ferulic acid markedly inhibits tumor volume and tumor weight, as compare to the counterparts treated with DMSO. Furthermore, Ferulic acid treatment is well tolerated, and there is no significant difference in weight between the vehicle group and the FA-treated groups[1]. Ferulic acid (0.01, 0.1, 1 or 10 mg/kg) given by oral route decreases significantly the immobility time in the forced swimming test (FST) and tail suspension test (TST), whereas produces no effect in the open-field test. Results demonstrate that the administration of Ferulic acid (0.001 mg/kg, p.o.) boosts the antidepressant-like effect of fluoxetine (5 mg/kg, p.o.) in the TST[2]. |
| Cell Assay | HUVEC (5×104 cells/well) are plated onto a gelatinized 24-well culture plate and cultured in ECGS containing 15% FBS. HUVEC are treated with DMSO (0.1%) or different concentrations of Ferulic acid (FA) (0, 2.5, 5, 10, 20, 30, 40 μM) for 24 h. Cell viability is determined by the MTT assay. After 4 h of incubation, the absorbance is measured at 450 nm with a microplate reader. The results are calculated from six replicates of each experiment. Three independent experiments are performed[1]. |
| Animal Admin | Male Swiss mice (30 to 40 g) are maintained at 21 to 23°C with free access to water and food, under a 12:12 h light/dark cycle (lights on at 07:00 h). All manipulations are carried out between, 9:00 and 16:00 h, with each animal used only once. In order to investigate the antidepressant-like effect of Ferulic acid, Ferulic acid is administered at a dose range of 0.001 to 10 mg/kg, by oral route (p.o.) 60 min before the forced swimming test (FST), tail suspension test (TST) or open-field test. The control animals receive appropriate vehicle[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 372.3±27.0 °C at 760 mmHg |
| Melting Point | 168-172 °C(lit.) |
| Molecular Formula | C10H10O4 |
| Molecular Weight | 194.184 |
| Flash Point | 150.5±17.2 °C |
| Exact Mass | 194.057907 |
| PSA | 66.76000 |
| LogP | 1.64 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.627 |
| Storage condition | -20°C Freezer |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UD3365500 |
| HS Code | 1302199099 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Characterization of p-hydroxycinnamate catabolism in a soil Actinobacterium.
J. Bacteriol. 196(24) , 4293-303, (2014) p-Hydroxycinnamates, such as ferulate and p-coumarate, are components of plant cell walls and have a number of commercial applications. Rhodococcus jostii RHA1 (RHA1) catabolizes ferulate via vanillat... |
|
|
[Simultaneous determination of five organic acids in Kudiezi injection by HPLC].
Zhongguo Zhong Yao Za Zhi 38(19) , 3287-90, (2013) The aim was to develop a high performance liquid chromatography method for simultaneous determination of five organic acids in Kudiezi injection. The Diamonsil C18 column (4.6 mm x 200 mm, 5 microm) w... |
|
|
Spectroscopic studies on the in vitro antioxidant capacity of isopentyl ferulate.
Chem. Biol. Interact. 225 , 47-53, (2015) Essential oils have played a prominent role in research on natural products, due to the high level of bioactive constituents, which include those derived from phenylpropanoids or terpenoids. This stud... |
| FERULLIC ACID |
| FERULIC ACID pure |
| Fumalic acid |
| conifericacid |
| Forulic acid |
| 3-(4-Hydroxy-3-methoxyphenyl)acrylic acid |
| Ferulic acid |
| 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)- |
| Ferulicacid |
| EINECS 208-679-7 |
| 4-hydroxy-3-methoxy-cinnamic acid |
| 4-Hydroxy-3-methoxycinnamic acid |
| MFCD00004400 |
| ferulaic acid |
 CAS#:141-82-2
CAS#:141-82-2 CAS#:121-33-5
CAS#:121-33-5 CAS#:4046-02-0
CAS#:4046-02-0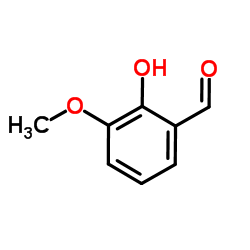 CAS#:148-53-8
CAS#:148-53-8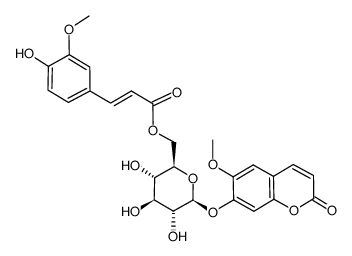 CAS#:85011-57-0
CAS#:85011-57-0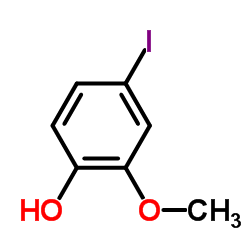 CAS#:203861-62-5
CAS#:203861-62-5 CAS#:79-10-7
CAS#:79-10-7 CAS#:823788-11-0
CAS#:823788-11-0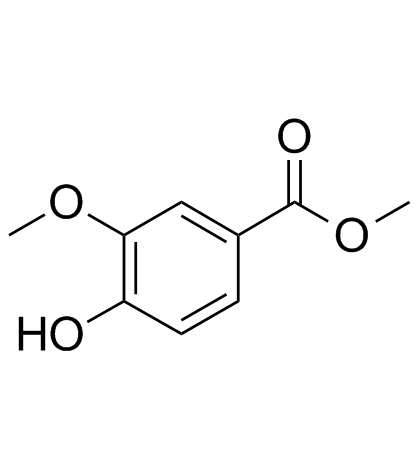 CAS#:3943-74-6
CAS#:3943-74-6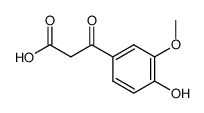 CAS#:84272-48-0
CAS#:84272-48-0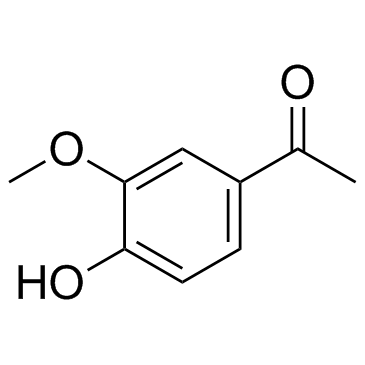 CAS#:498-02-2
CAS#:498-02-2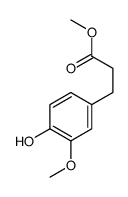 CAS#:56024-44-3
CAS#:56024-44-3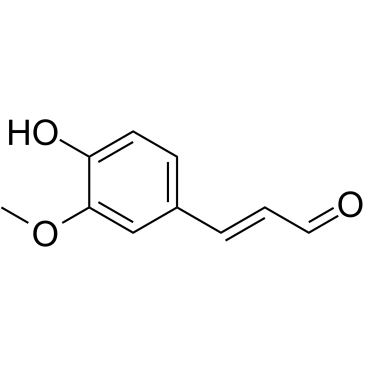 CAS#:458-36-6
CAS#:458-36-6 CAS#:90843-62-2
CAS#:90843-62-2 CAS#:458-35-5
CAS#:458-35-5 CAS#:19272-90-3
CAS#:19272-90-3
