(3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane
Modify Date: 2024-01-10 11:28:03
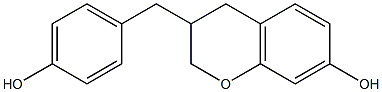
(3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane structure
|
Common Name | (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane | ||
|---|---|---|---|---|
| CAS Number | 1180504-64-6 | Molecular Weight | 256.29644 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H16O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane(3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane is a homoisoflavonoid. (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane increases the level of alkaline phosphatase (ALP) activity. (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane promotes mesenchymal stem cells (MSCs) osteogenesis, but cannot enhance MSCs proliferation. (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane can be used for osteoporosis research[1]. |
| Name | 7-Hydroxy-3-(4-hydroxybenzyl)chroman |
|---|
| Description | (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane is a homoisoflavonoid. (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane increases the level of alkaline phosphatase (ALP) activity. (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane promotes mesenchymal stem cells (MSCs) osteogenesis, but cannot enhance MSCs proliferation. (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane can be used for osteoporosis research[1]. |
|---|---|
| Related Catalog | |
| Target |
Alkaline phosphatase (ALP)[1] |
| In Vitro | (3R)-7-hydroxy-3-(4-hydroxybenzyl)chromane (10 μM) 通过增加碱性磷酸酶 (ALP) 活性水平显著促进 MSCs 成骨分化。与对照组相比,早期成骨细胞分化率为162.0±1.4[1]。 |
| References |
| Molecular Formula | C16H16O3 |
|---|---|
| Molecular Weight | 256.29644 |