(-)-JQ-1
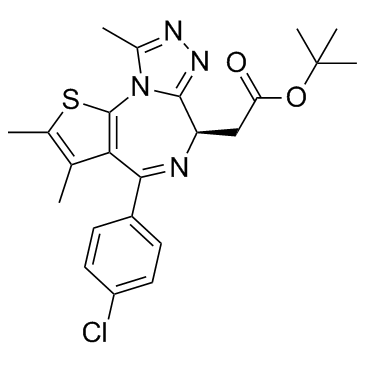
(-)-JQ-1 structure
|
Common Name | (-)-JQ-1 | ||
|---|---|---|---|---|
| CAS Number | 1268524-71-5 | Molecular Weight | 456.988 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 610.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C23H25ClN4O2S | Melting Point | N/A | |
| MSDS | USA | Flash Point | 322.9±34.3 °C | |
Use of (-)-JQ-1(-)-JQ-1 is the stereoisomer of (+)-JQ1. (+)-JQ1 potently decreases expression of both BRD4 target genes, whereas (−)-JQ1 has no effect. |
| Name | ()-jq1 |
|---|---|
| Synonym | More Synonyms |
| Description | (-)-JQ-1 is the stereoisomer of (+)-JQ1. (+)-JQ1 potently decreases expression of both BRD4 target genes, whereas (−)-JQ1 has no effect. |
|---|---|
| Related Catalog | |
| Target |
BET bromodomain[1] |
| In Vitro | (−)-JQ1 shows no significant interaction with any bromodomain. Besides, (−)-JQ1 enantiomer is comparatively inactive in nuclear protein in testis (NUT) midline carcinoma (NMC)[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 610.4±65.0 °C at 760 mmHg |
| Molecular Formula | C23H25ClN4O2S |
| Molecular Weight | 456.988 |
| Flash Point | 322.9±34.3 °C |
| Exact Mass | 456.138672 |
| PSA | 97.61000 |
| LogP | 4.49 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.657 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
Targeting bromodomains: epigenetic readers of lysine acetylation.
Nat. Rev. Drug Discov. 13(5) , 337-56, (2014) Lysine acetylation is a key mechanism that regulates chromatin structure; aberrant acetylation levels have been linked to the development of several diseases. Acetyl-lysine modifications create dockin... |
|
|
Epigenetic chemical probes. Müller S and Brown PJ
Clin. Pharmacol. Ther. 92(6) , 689-93, (2012)
|
|
|
Targeting chromatin readers. James LI and Frye SV
Clin. Pharmacol. Ther. 93(4) , 312-4, (2013)
|
| 6H-Thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-6-acetic acid, 4-(4-chlorophenyl)-2,3,9-trimethyl-, 1,1-dimethylethyl ester, (6R)- |
| 2-Methyl-2-propanyl [(6R)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]acetate |
| (R)-(-)-JQ1 Enantiomer |

