Cercosporamide
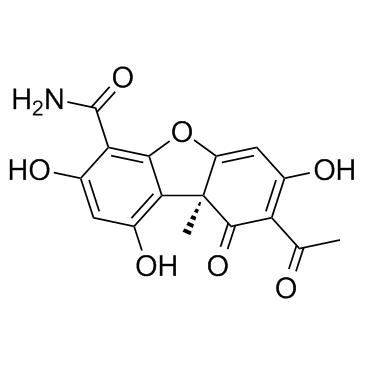
Cercosporamide structure
|
Common Name | Cercosporamide | ||
|---|---|---|---|---|
| CAS Number | 131436-22-1 | Molecular Weight | 331.277 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 582.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H13NO7 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 306.1±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CercosporamideCercosporamide is a highly potent, ATP-competitive Pkc1 kinase inhibitor, with an IC50 of <50 nM and a Ki of <7 nM. Cercosporamide is a unique Mnk inhibitor. |
| Name | cercosporamide |
|---|---|
| Synonym | More Synonyms |
| Description | Cercosporamide is a highly potent, ATP-competitive Pkc1 kinase inhibitor, with an IC50 of <50 nM and a Ki of <7 nM. Cercosporamide is a unique Mnk inhibitor. |
|---|---|
| Related Catalog | |
| Target |
Pkc1:50 nM (IC50) Pkc1:7 nM (Ki) Mnk |
| In Vitro | Cercosporamide is a broad-spectrum natural antifungal compound, is actually a selective and highly potent fungal Pkc1 kinase inhibitor[1]. Cercosporamide, an antifungal agent that is recently shown to act as a unique Mnk inhibitor, exhibits antileukemic properties. Cercosporamide is a potent inhibitor of phosphorylation of eIF4E at Ser209 in AML cells and results in potent inhibitory effects on primitive leukemic progenitors (CFU-L) from AML patients. To determine whether Cercosporamide exhibits negative regulatory effects on cell proliferation and viability of leukemia cells, MTT assays are conducted. When U937 cells are incubated in the presence or absence of the increasing doses of Cercosporamide, a dose-dependent suppression of cell growth is found. Similar experiments with comparable results are seen when the effects of Cercosporamide on MM6 and K562 cells are examined[2]. |
| In Vivo | Treatment with Cercosporamide or Ara-C alone significantly suppresses xenograft growth when compared with the respective vehicle (P<0.011 for 10 mg/kg twice-daily Cercosporamide; P<0.006 for Cercosporamide 20 mg/kg daily; P<0.0374 for Ara-C). The combination of Cercosporamide 10 mg/kg twice daily plus Ara-C is significantly more effective than either agent alone (P<0.0009 vs Cercosporamide; P=0.005 vs Ara-C; P<0.0001 vs either vehicle). Cercosporamide (20 mg/kg once daily) in combination with Ara-C shows similar effects, with significant inhibition of tumor growth vs captisol (P<0.0001) or water (P=0.0003), but does not show statistical significance vs Cercosporamide alone (20 mg/kg) or Ara-C alone[2]. |
| Cell Assay | U937, MM6, and K562 cells are incubated for 5 days in the presence or absence of the indicated doses of Cercosporamide (1, 10, and 20μM) . Cell proliferation is assessed by an MTT assay[2]. |
| Animal Admin | Mice[2] MV4-11 cells are implanted at a density of 5×106 cells per mouse. Tumors are measured by caliper and tumor volume is calculated. Once tumors reach a group mean of 100 mm3, animals are randomized to the following treatment groups: Ara-C (20 mg/kg daily dosed intraperitoneally), Cercosporamide (10 mg/kg twice daily, 20 mg/kg daily dosed orally by gavage), Ara-C plus Cercosporamide combinations (as above), or the relative vehicle controls (captisol for Cercosporamide and water for Ara-C)[2]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 582.5±50.0 °C at 760 mmHg |
| Molecular Formula | C16H13NO7 |
| Molecular Weight | 331.277 |
| Flash Point | 306.1±30.1 °C |
| Exact Mass | 331.069214 |
| PSA | 148.14000 |
| LogP | 0.00 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.741 |
| Storage condition | 2-8℃ |
|
Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens.
Appl. Microbiol. Biotechnol. 93(3) , 1231-9, (2012) Through bioassay-guided fractionation, the EtOAc extract of a culture broth of the endophytic fungus Phoma species ZJWCF006 in Arisaema erubescens afforded a new α-tetralone derivative, (3S)-3,6,7-tri... |
|
|
Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors.
Blood 121(18) , 3675-81, (2013) Mnk kinases regulate the phosphorylation and activation of the eukaryotic initiation factor 4E (eIF4E), a protein that plays key roles in the initiation of messenger RNA translation and whose activity... |
|
|
Cloning and characterization of KNR4, a yeast gene involved in (1,3)-beta-glucan synthesis.
Mol. Cell. Biol. 14(2) , 1017-25, (1994) k9 killer toxin from Hansenula mrakii was used to select a number of resistant mutants from Saccharomyces cerevisiae. Preliminary biochemical and genetic studies showed that some of them acquired stru... |
| 4-Dibenzofurancarboxamide, 8-acetyl-9,9a-dihydro-1,3,7-trihydroxy-9a-methyl-9-oxo-, (9aS)- |
| 4-Dibenzofurancarboxamide |
| (9aS)-8-acetyl-1,3,7-trihydroxy-9a-methyl-9-oxodibenzofuran-4-carboxamide |
| (9aS)-8-Acetyl-1,3,7-trihydroxy-9a-methyl-9-oxo-9,9a-dihydrodibenzo[b,d]furan-4-carboxamide |