Cytarabine
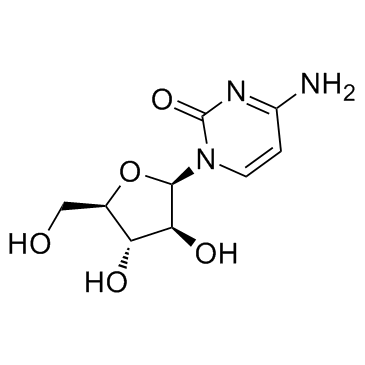
Cytarabine structure
|
Common Name | Cytarabine | ||
|---|---|---|---|---|
| CAS Number | 147-94-4 | Molecular Weight | 243.217 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 529.7±60.0 °C at 760 mmHg | |
| Molecular Formula | C9H13N3O5 | Melting Point | 214 °C | |
| MSDS | Chinese USA | Flash Point | 274.1±32.9 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of CytarabineCytarabine is a chemotherapy drug used to treat acute myeloid leukaemia, acting by inhibiting DNA synthesis with an IC50 of 16 nM. |
| Name | cytarabine |
|---|---|
| Synonym | More Synonyms |
| Description | Cytarabine is a chemotherapy drug used to treat acute myeloid leukaemia, acting by inhibiting DNA synthesis with an IC50 of 16 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 16 nM (DNA synthesis) |
| In Vitro | Cytarabine is phosphorylated into a triphosphate form (Ara-CTP) involving deoxycytidine kinase (dCK), which competes with dCTP for incorporation into DNA, and then blocks DNA synthesis by inhibiting the function of DNA and RNA polymerases. Cytarabine displays a higher growth inhibitory activity towards wild-type CCRF-CEM cells compared to other acute myelogenous leukemia (AML) cells with IC50 of 16 nM[1]. Cytarabine apparently induces apoptosis of rat sympathetic neurons at 10 μM, of which 100 μM shows the highest toxicity and kills over 80% of the neurons by 84 hours, involving the release of mitochondrial cytochrome-c and the activation of caspase-3, and the toxicity can be attenuated by p53 knockdown and delayed by bax deletion[2]. |
| In Vivo | Cytarabine (250 mg/kg) also causes placental growth retardation and increases placental trophoblastic cells apoptosis in the placental labyrinth zone of the pregnant Slc:Wistar rats, which increases from 3 hour after the treatment and peaks at 6 hour before returning to control levels at 48 hour, with remarkably enhanced p53 protein, p53 trancriptional target genes such as p21, cyclinG1 and fas and caspase-3 activity[3]. Cytarabine is highly effective against acute leukaemias, which causes the Cytarabine teristic G1/S blockage and synchronization, and increases the survival time for leukaemic Brown Norway rats in a weak dose-related fashion indicating that the use of higher dosages of Cytarabine does not contribute to its antileukaemic effectiveness in man[4]. |
| Kinase Assay | Stock solution of Cytarabine is prepared in absolute ethanol, and serial dilutions of Cytarabine are prepared. CCRF-CEM cells are suspended in RPMI medium supplemented with 10% FBS, 0.1% gentamicin, and 1% sodium pyruvate. The cells are suspended in their respective media to give 10 mL volumes of cell suspension at a final density of 3-6×104 cells/mL. Appropriate volumes of Cytarabine solution are transferred to the cell suspensions, and incubation is continued for 72 hours. The cells are spun down and resuspended in fresh Cytarabine -free medium, and final cell counts are determined. The data are analyzed by sigmoidal curve fitting of the cell count versus Cytarabine concentration, and the results are expressed as the IC50 (Cytarabine concentration that inhibits cell growth to 50% of the control value). |
| Animal Admin | Pregnant rats are injected intraperitoneally (i.p.) with 250 mg/kg of Cytarabine on Day 13 of gestation (GD13). Under the conditions of this experiment, congenital anomalies and growth retardation are detected at a high rate in perinatal fetuses, although the incidence of fetal death is not markedly increased. At 1, 3, 6, 9, 12, 24, and 48 h after the treatment, six dams each are killed by heart puncture under ether anesthesia, and the placentas are collected. As controls, six pregnant rats are injected i.p. with an equivalent volume of PBS on GD13 and killed at the same time point as Cytarabine-treated groups. Of the six dams obtained at each time point, three are used for histopathological analyses and three for reverse transcription-polymerase chain reaction (RT-PCR) analysis. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 529.7±60.0 °C at 760 mmHg |
| Melting Point | 214 °C |
| Molecular Formula | C9H13N3O5 |
| Molecular Weight | 243.217 |
| Flash Point | 274.1±32.9 °C |
| Exact Mass | 243.085526 |
| PSA | 130.83000 |
| LogP | -1.78 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.756 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 50 mg/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H361 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R43;R63 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HA5425000 |
| HS Code | 2934999090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Retinoic acid synergizes ATO-mediated cytotoxicity by precluding Nrf2 activity in AML cells.
Br. J. Cancer 111(5) , 874-82, (2014) Standard therapy for acute promyelocytic leukaemia (APL) includes retinoic acid (all-trans retinoic acid (ATRA)), which promotes differentiation of promyelocytic blasts. Although co-administration of ... |
|
|
African swine fever virus ORF P1192R codes for a functional type II DNA topoisomerase.
Virology 474 , 82-93, (2014) Topoisomerases modulate the topological state of DNA during processes, such as replication and transcription, that cause overwinding and/or underwinding of the DNA. African swine fever virus (ASFV) is... |
|
|
PMP22 is critical for actin-mediated cellular functions and for establishing lipid rafts.
J. Neurosci. 34(48) , 16140-52, (2014) Haploinsufficiency of peripheral myelin protein 22 (PMP22) causes hereditary neuropathy with liability to pressure palsies, a peripheral nerve lesion induced by minimal trauma or compression. As PMP22... |
| MFCD00066487 |
| Cytosine β-D-Arabinofuranoside |
| Cytarabine |
| Arabinosylcytosine |
| 4-Amino-1-β-D-arabinofuranosyl-2(1H)-pyrimidinone |
| Arabinocytidine |
| 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one |
| (β-D-Arabinofuranosyl)cytosine |
| EINECS 205-705-9 |
| 4-Amino-1-((2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one |
| Arabinofuranosylcytosine |
| (β-D-Arabinofuranosyl)cytosine |
 CAS#:10212-25-6
CAS#:10212-25-6 CAS#:6742-07-0
CAS#:6742-07-0 CAS#:3083-77-0
CAS#:3083-77-0 CAS#:82855-62-7
CAS#:82855-62-7 CAS#:1137985-55-7
CAS#:1137985-55-7 CAS#:25130-27-2
CAS#:25130-27-2 CAS#:31698-14-3
CAS#:31698-14-3 CAS#:170935-57-6
CAS#:170935-57-6 CAS#:66-22-8
CAS#:66-22-8 CAS#:69-74-9
CAS#:69-74-9 CAS#:7075-11-8
CAS#:7075-11-8 CAS#:34079-68-0
CAS#:34079-68-0 CAS#:62-49-7
CAS#:62-49-7 CAS#:85145-70-6
CAS#:85145-70-6 CAS#:107612-33-9
CAS#:107612-33-9 CAS#:13491-47-9
CAS#:13491-47-9![[3,4-diacetyloxy-5-(4-amino-2-oxo-pyrimidin-1-yl)oxolan-2-yl]methyl acetate structure](https://image.chemsrc.com/caspic/421/58227-71-7.png) CAS#:58227-71-7
CAS#:58227-71-7
