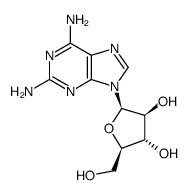
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
HA5425000
-
CHEMICAL NAME :
-
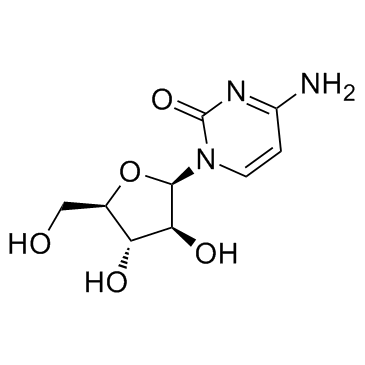
Cytosine, 1-beta-D-arabinofuranosyl-
-
CAS REGISTRY NUMBER :
-
147-94-4
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
122
-
MOLECULAR FORMULA :
-
C9-H13-N3-O5
-
MOLECULAR WEIGHT :
-
243.25
-
WISWESSER LINE NOTATION :
-
T6NVNJ DZ A- BT5OTJ CQ DQ E1Q
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Human
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration into the eye
-
SPECIES OBSERVED :
-
Human
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
60 mg/kg/90W-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Ear) - change in acuity Behavioral - ataxia Blood - changes in spleen
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
6480 ug/kg/12D-I
-
TOXIC EFFECTS :
-
Blood - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
33200 ug/kg/240D-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
17241 mg/kg/6D-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, allergic (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
720 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Brain and Coverings - other degenerative changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
649 mg/kg/4D-I
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - fasciculations
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1536 mg/kg/43W-I
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - fasciculations Behavioral - ataxia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
23500 ug/kg/7D-C
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - visual field changes Sense Organs and Special Senses (Eye) - lacrimation Sense Organs and Special Senses (Eye) - conjunctive irritation
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3150 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3779 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>7 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
875 mg/kg/5W-I
-
TOXIC EFFECTS :
-
Endocrine - changes in spleen weight Blood - changes in erythrocyte (RBC) count Blood - changes in leukocyte (WBC) count
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
300 mg/kg/5D-I
-
TOXIC EFFECTS :
-
Blood - changes in bone marrow (not otherwise specified) Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
280 mg/kg/7D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Blood - changes in erythrocyte (RBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
280 mg/kg/7D-I
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Gastrointestinal - hypermotility, diarrhea Blood - changes in erythrocyte (RBC) count
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2500 mg/kg/7W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - lymphoma, including Hodgkin's disease Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4836 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Lungs, Thorax, or Respiration - tumors Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
7500 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
75 mg/kg
-
SEX/DURATION :
-
female 18-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
90 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
360 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
180 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - cytological changes (including somatic cell genetic material)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 13-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
33 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 13-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
80 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
45 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Effects on Newborn - live birth index (measured after birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
18 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
9 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 13-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
Specific locus test
-
TYPE OF TEST :
-
Specific locus test
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TYPE OF TEST :
-
Dominant lethal test
-
TYPE OF TEST :
-
Sperm Morphology
-
TYPE OF TEST :
-
DNA inhibition
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Mammal - species unspecified Fibroblast
-
DOSE/DURATION :
-
15 umol/L
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 178,73,1987 *** REVIEWS *** TOXICOLOGY REVIEW 32XPAD "Teratology," Berry, C.L., and D.E. Poswillo, eds., New York, Springer, 1975 Volume(issue)/page/year: -,49,1975 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW CRTXB2 CRC Critical Reviews in Toxicology. (CRC Press, Inc., 2000 Corporate Blvd., NW, Boca Raton, FL 33431) V.1- 1971- Volume(issue)/page/year: 2,159,1973 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X3689 No. of Facilities: 450 (estimated) No. of Industries: 1 No. of Occupations: 7 No. of Employees: 18673 (estimated) No. of Female Employees: 12859 (estimated)
|






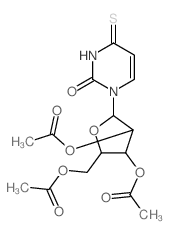


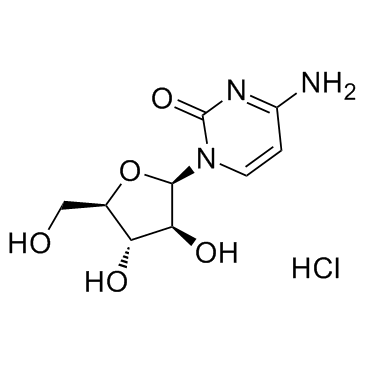


![[3,4-diacetyloxy-5-(4-amino-2-oxo-pyrimidin-1-yl)oxolan-2-yl]methyl acetate structure](https://image.chemsrc.com/caspic/421/58227-71-7.png)