3-Carbamimidamido-L-alanine hydrochloride (1:1)
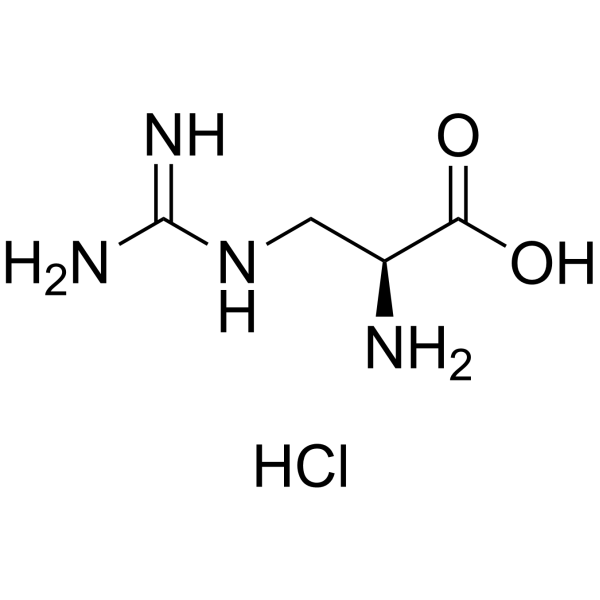
3-Carbamimidamido-L-alanine hydrochloride (1:1) structure
|
Common Name | 3-Carbamimidamido-L-alanine hydrochloride (1:1) | ||
|---|---|---|---|---|
| CAS Number | 1482-99-1 | Molecular Weight | 182.609 | |
| Density | N/A | Boiling Point | 370.8ºC at 760 mmHg | |
| Molecular Formula | C4H11ClN4O2 | Melting Point | -220ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 178.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 3-Carbamimidamido-L-alanine hydrochloride (1:1)(S)-2-amino-3-guanidinopropanoic acid hydrochloride is an alanine derivative[1]. |
| Name | L-2-Amino-3-guanidinopropionic acid hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-2-amino-3-guanidinopropanoic acid hydrochloride is an alanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Boiling Point | 370.8ºC at 760 mmHg |
|---|---|
| Melting Point | -220ºC (dec.) |
| Molecular Formula | C4H11ClN4O2 |
| Molecular Weight | 182.609 |
| Flash Point | 178.1ºC |
| Exact Mass | 182.057053 |
| PSA | 125.22000 |
| LogP | 0.57460 |
| Vapour Pressure | 1.62E-06mmHg at 25°C |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~% 
3-Carbamimidami... CAS#:1482-99-1 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 7, p. 616 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Noncoded amino acid replacement probes of the aspartate aminotransferase mechanism.
Biochemistry 36 , 10517-10525, (1997) The primary role of Tyr225 in the aspartate aminotransferase mechanism is to provide a hydrogen bond to stabilize the 3'O- functionality of bound pyridoxal phosphate. The strength of this hydrogen bon... |
|
|
Binding of the unreactive substrate analog L-2-amino-3-guanidinopropionic acid (dinor-L-arginine) to human arginase I.
Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 68(Pt 8) , 889-93, (2012) Human arginase I (HAI) is a binuclear manganese metalloenzyme that catalyzes the hydrolysis of L-arginine to form L-ornithine and urea through a metal-activated hydroxide mechanism. Since HAI regulate... |
|
|
Analysis of 1- and 3-methylhistidines, aromatic and basic amino acids in rat and human urine. Feldhoff RC, Ledden DJ, et al.
J. Chromatogr. A. 311 , 267-276, (1984)
|
| Ethanaminium, 1-carboxy-2-[(diaminomethylene)amino]-, chloride (1:1) |
| 1-Carboxy-2-[(diaminomethylene)amino]ethanaminium chloride |
| L-Alanine, 3-[(aminoiminomethyl)amino]-, hydrochloride (1:1) |
| (S)-2-Amino-3-guanidinopropanoic acid hydrochloride |
| L-2-Amino-3-guanidinopropionic acid hydrochloride |
| 3-Guanidino-L-alanine hydrochloride |
| 3-Carbamimidamido-L-alanine hydrochloride (1:1) |
| (2S)-2-amino-3-(diaminomethylideneamino)propanoic acid,hydrochloride |


