Cisplatin
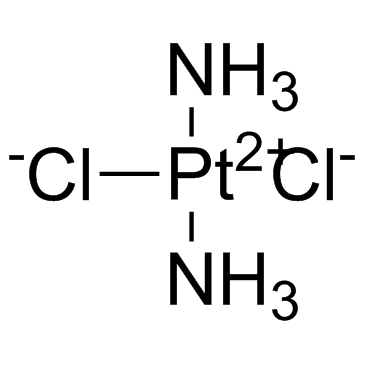
Cisplatin structure
|
Common Name | Cisplatin | ||
|---|---|---|---|---|
| CAS Number | 15663-27-1 | Molecular Weight | 300.05 | |
| Density | 3.7 | Boiling Point | N/A | |
| Molecular Formula | Cl2H6N2Pt | Melting Point | 270ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |



GHS05, GHS06, GHS08 |
Signal Word | Danger | |
Use of CisplatinCisplatin is a antineoplastic chemotherapy drug which works by cross-linking with DNA and causing DNA damage in cancer cells. |
| Name | cisplatin |
|---|---|
| Synonym | More Synonyms |
| Description | Cisplatin is a antineoplastic chemotherapy drug which works by cross-linking with DNA and causing DNA damage in cancer cells. |
|---|---|
| Related Catalog | |
| Target |
DNA Alkylator/Crosslinker[1] |
| In Vitro | Cisplatin (CDDP) causes apoptosis of HeLa cells in a dose-dependent manner, with a concentration of 30 μM Cisplatin resulting in death of greater than 90% of the cell population by 24 h of treatment. The kinetics of Cisplatin-induced apoptosis are examined using a 30 μM concentration. Cisplatin Activates the MEK/ERK Signaling Pathway, 20 and 30 μM Cisplatin, both of which results in significant apoptosis, leads to strong activation of ERK[1]. Cisplatin (50 μM) produces time-dependent apoptosis in renal proximal tubular cell (RPTCs), causing cell shrinkage, a 50-fold increase in caspase 3 activity, a 4-fold increase in phosphatidylserine externalization, and 5- and 15-fold increases in chromatin condensation and DNA hypoploidy, respectively[2]. |
| In Vivo | In melanoma-bearing mice, Cisplatin (4 mg/kg B.W.) reduces the size and weight of the solid tumors, and HemoHIM supplementation with Cisplatin enhances the decrease of both the tumor size and weight[3]. Cisplatin administration results in significant increases in the kidney weight as a percentage of the total body weight, urine volume, serum creatinine, and blood urea nitrogenby about 132, 315, 797, and 556% in comparison with the control rats, respectively[4]. |
| Cell Assay | HeLa and A549 cells are maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units of Penicillin, and 100 μg of Streptomycin/mL. They are cultured at 37°C in a humidified chamber containing 5% CO2. For the induction of apoptosis, cells are plated in 60-mm dishes 1 day prior to Cisplatin (0-30 μM) treatment[1]. |
| Animal Admin | Mice[3] Mice are divided randomly into three groups (Control, Cisplatin and Cisplatin+HemoHIM), and each group consists of twenty mice. B16F0 melanoma (5×105 cells/mouse) is inoculated into subcutaneous femoral left region of mice at 3 days before an initial injection of Cisplatin. Cisplatin is injected intraperitoneally at 4 mg/kg body weight (B.W.) on day 0, 7 and 14 (total three injections). Experimental group is intubated with HemoHIM at a final concentration of 100 mg/kgB.W. by everyday from day -1 to day 16, while the control group received only water. On day 17 after initial injection of Cisplatin, all mice of each group are experimented, respectively, to evaluate tumor weight or tumor size. The tumor size is calculated as follows: tumor size=ab2/2, where a and b are the larger and smaller diameters, respectively. Rats[4] Male Sprague-Dawley rats weighing 200 to 250 g are divided at random into 4 groups of 4 or 5 animals each. The first group (control) received a vehicle (5% carboxymethyl cellulose sodium solution (CMC-Na), 5 mL/kg body wt., p.o.) used for Capsaicin (Cap). The second group received Cap (10 mg/kg/d, p.o.) in 5% CMC-Na (5 mL/kg), and the third received 5% CMC-Na for 6 consecutive days injected with Cisplatin (5 mg/kg in physiological saline solution, i.p.). The fourth group received Cap (10 mg/kg/d, p.o.) in 5% CMC-Na for 6 consecutive days after Cisplatin injection (5 mg/kg, i.p.). For all groups, Cap or vehicle is given twice daily. The selected Cap concentration and the dose administration schedule without inducing any rat intestinal damage are chosen using data from our preliminary experiments. |
| References |
| Density | 3.7 |
|---|---|
| Melting Point | 270ºC |
| Molecular Formula | Cl2H6N2Pt |
| Molecular Weight | 300.05 |
| PSA | 52.04000 |
| LogP | 1.59590 |
| Storage condition | Store at RT |
| Stability | Stable. Incompatible with oxidizing agents, aluminium, antioxidants. |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Symbol |



GHS05, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H318-H350 |
| Precautionary Statements | P201-P264-P280-P301 + P310-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R45;R25;R41 |
| Safety Phrases | S53-S26-S39-S45 |
| RIDADR | UN 1851/3288 |
| WGK Germany | 3 |
| RTECS | TP2455000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2932999099 |
| Precursor 0 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
TAp73 promotes cell survival upon genotoxic stress by inhibiting p53 activity.
Oncotarget 5(18) , 8107-22, (2014) p53 plays a key role in regulating DNA damage response by suppressing cell cycle progression or inducing apoptosis depending on extent of DNA damage. However, it is not clear why mild genotoxic stress... |
|
|
Combination of systemic chemotherapy with local stem cell delivered S-TRAIL in resected brain tumors.
Stem Cells 33(1) , 101-10, (2014) Despite advances in standard therapies, the survival of glioblastoma multiforme (GBM) patients has not improved. Limitations to successful translation of new therapies include poor delivery of systemi... |
|
|
Crystals of Na(+)/K(+)-ATPase with bound cisplatin.
Biochem. Pharmacol. 92(3) , 494-8, (2014) Cisplatin is the most widely used chemotherapeutics for cancer treatment, however, its administration is connected to inevitable adverse effects. Previous studies suggested that cisplatin is able to i... |
| trans--diamminedichloroplatinum(II) |
| trans-diamminodichloroplatinum(II) |
| UNII:Q20Q21Q62J |
| EINECS 239-733-8 |
| Dichloroplatinum diammoniate |
| cis-Diamminedichloroplatinum(II) |
| cis-Diamineplatinum(II) dichloride |
| cis-Platinum(II) diammine dichloride |
| MFCD00011623 |
| transplatin |
| trans-dichlorodiammineplatinum(II) |
| trans-dichlorodiamine platinum(II) |
| cis-Diammineplatinum(II) dichloride (cis-Dichlorodiammine platinum(II) |
| trans-Diamminedichloroplatinum(II) (trans-Dichlorodiammineplatinum(II) |
| cis-Diamminedichlorplatine |
| cis-Dichlorodiammine platinum(II) |
| cis-Dichlorodiamineplatinum(II) |
| cis-Diammineplatinum dichloride |
| trans-diaminedichloroplatinum(II) |
| cis-Diammineplatinum(II) dichloride |
| Cisplatin |
| trans-Platinum diammine dichloride |
| CIS-PLATIN |
| trans-Dichlorodiamineplatinum(II) |
| Cisplatin impurity A |
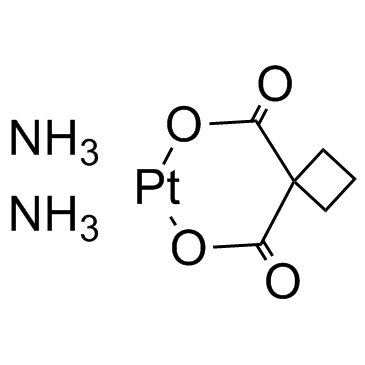 CAS#:41575-94-4
CAS#:41575-94-4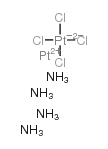 CAS#:13820-46-7
CAS#:13820-46-7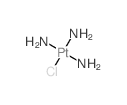 CAS#:13815-16-2
CAS#:13815-16-2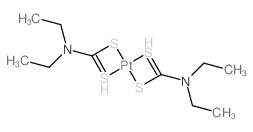 CAS#:15730-38-8
CAS#:15730-38-8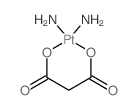 CAS#:38780-43-7
CAS#:38780-43-7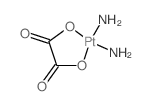 CAS#:41349-15-9
CAS#:41349-15-9![Platinum,diammine[hydroxypropanedioato(2-)-kO1,kO3]-, (SP-4-2)- (9CI) structure](https://image.chemsrc.com/caspic/232/52260-82-9.png) CAS#:52260-82-9
CAS#:52260-82-9 CAS#:7647-01-0
CAS#:7647-01-0 CAS#:7727-37-9
CAS#:7727-37-9 CAS#:7664-41-7
CAS#:7664-41-7
