Carboplatin
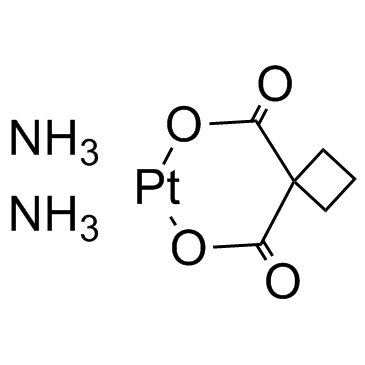
Carboplatin structure
|
Common Name | Carboplatin | ||
|---|---|---|---|---|
| CAS Number | 41575-94-4 | Molecular Weight | 371.254 | |
| Density | N/A | Boiling Point | 366.4ºCat 760 mmHg | |
| Molecular Formula | C6H12N2O4Pt | Melting Point | 228-230ºC | |
| MSDS | Chinese USA | Flash Point | 189.6ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of CarboplatinCarboplatin (NSC 241240) is a DNA synthesis inhibitor which binds to DNA, inhibits replication and transcription and induces cell death. Carboplatin (NSC 241240) is a derivative of cisplatin and a potent anti-cancer agent. |
| Name | carboplatin |
|---|---|
| Synonym | More Synonyms |
| Description | Carboplatin (NSC 241240) is a DNA synthesis inhibitor which binds to DNA, inhibits replication and transcription and induces cell death. Carboplatin (NSC 241240) is a derivative of cisplatin and a potent anti-cancer agent. |
|---|---|
| Related Catalog | |
| Target |
DNA Alkylator[1] |
| In Vitro | Carboplatin is an antitumor agent, with an increased DNA-binding activity in the presence of nucleophiles and human breast cancer MCF-7 cell cytoplasmic extracts[1]. Carboplatin is less cytotoxic to human ovarian cells such as A2780, SKOV3, IGROV1 and HX62 than 17-AAG, with IC50s of 6.177, 12.442, 2.233 and 116.068 μM, respectively. Moreover, Carboplatin does not affect HSP90 or change the activity of 17-AAG to inhibit HSP90[2]. Carboplatin reduces the viability of Brca1 (IC50, 3.4 μM) and Brca2 cells (IC50, 1.9 μM). Carboplatin (25 μM) combined with ABT-888 also shows an apoptotic effect in BRCA1 cells[3]. |
| In Vivo | Carboplatin (60 mg/kg, i.p.) shows a modest effect on the tumor, but significantly inhibits tumor growth in combination with 17-AAG in mice bearing A2780 human ovarian cancer xenografts[2]. Carboplatin (25 mg/kg, p.o.) combined with ABT-888 delays tumor growth in Brca2 xenografts[3]. |
| Cell Assay | Exponentially growing A2780 cells are plated in 96 well microtitre plates. For experiments studying the effect of sequence of exposure to 17-AAG or Carboplatin, cells are exposed to increasing concentrations of 17-AAG or Carboplatin for 24 h. A period of 24-h exposure to the first agent is chosen so that the A2780 cells will be exposed to the first drug for at least one doubling time (18-24 h). The cells are then washed with sterile phosphate buffered saline and the medium is replenished. Following this, the second drug (to which the cells are not exposed to in the first 24 h) or medium is added for 96 h. SRB assays are carried out. All experiments are carried out in triplicate[2]. |
| Animal Admin | Mice[2] The A2780 human ovarian cancer cell line is grown as a subcutaneous xenograft in female athymic NCr nude mice (nu/nu) by injecting 4 × 106 cells in each flank. Mice with established tumors corresponding to a mean volume of 0.69 mm3 are randomized into groups (six animals each) for treatment with either control vehicle (43% ethanol, 33% polypropylene glycol and 24% cremaphor diluted 1:7 with sterile water) days 1-4, 17-AAG (80 mg/kg intraperitonially, days 1-4), Carboplatin (60 mg/kg IP day 0) or a combination of 17-AAG (80 mg/kg IP days 1-4) and Carboplatin (60 mg/kg IP day 0). Tumor growth is assessed three times weekly and tumor volumes are calculated according to a validated formula: volume = 4.19 × (a/4 + b/4)3, where a is the longer and b the shorter diameter. Tumor volumes are then expressed as a proportion of the volume at the start of treatment (relative tumor volume)[2]. |
| References |
| Boiling Point | 366.4ºCat 760 mmHg |
|---|---|
| Melting Point | 228-230ºC |
| Molecular Formula | C6H12N2O4Pt |
| Molecular Weight | 371.254 |
| Flash Point | 189.6ºC |
| Exact Mass | 371.044495 |
| PSA | 59.08000 |
| LogP | 0.81700 |
| Storage condition | Store at RT |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | Sparingly soluble in water, very slightly soluble in acetone and in ethanol (96 per cent). | Soluble in water. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302 + H312 + H332-H317-H334-H340-H360 |
| Precautionary Statements | P201-P261-P280-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R46;R61;R20/21;R42/43 |
| Safety Phrases | S53-S22-S26-S36/37/39-S45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | TP2300000 |
| HS Code | 2843900030 |
|
~68% 
Carboplatin CAS#:41575-94-4 |
| Literature: Pasini, Alessandro; Caldirola, Cristina Inorganica Chimica Acta, 1988 , vol. 151, p. 19 - 20 |
|
~% 
Carboplatin CAS#:41575-94-4 |
| Literature: Escribano, Esther; Font-Bardia, Merce; Calvet, Teresa; Lorenzo, Julia; Gamez, Patrick; Moreno, Virtudes Inorganica Chimica Acta, 2013 , vol. 394, p. 65 - 76 |
|
~65% 
Carboplatin CAS#:41575-94-4 |
| Literature: Pasini, Alessandro; Caldirola, Cristina Inorganica Chimica Acta, 1988 , vol. 151, p. 19 - 20 |
| HS Code | 2843900030 |
|---|
|
Mer590, a novel monoclonal antibody targeting MER receptor tyrosine kinase, decreases colony formation and increases chemosensitivity in non-small cell lung cancer.
Oncotarget 5(21) , 10434-45, (2014) The successes of targeted therapeutics against EGFR and ALK in non-small cell lung cancer (NSCLC) have demonstrated the substantial survival gains made possible by precision therapy. However, the majo... |
|
|
Graphene oxide - gelatin nanohybrids as functional tools for enhanced Carboplatin activity in neuroblastoma cells.
Pharm. Res. 32 , 2132-43, (2015) Preparation of Nanographene oxide (NGO) - Gelatin hybrids for efficient treatment of Neuroblastoma.Nanohybrids were prepared via non-covalent interactions. Spectroscopic tools have been used to discri... |
|
|
CD157 enhances malignant pleural mesothelioma aggressiveness and predicts poor clinical outcome.
Oncotarget 5(15) , 6191-205, (2014) Malignant mesothelioma is a deadly tumor whose diagnosis and treatment remain very challenging. There is an urgent need to advance our understanding of mesothelioma biology and to identify new molecul... |
| Diammine[1,1-cyclobutanedicarboxylato(2-)-κO,O]platinum |
| cis-Diammine(1,1-cyclobutanedicarboxylato) platinum |
| Platinum(2+) 1,1-cyclobutanedicarboxylate diammoniate |
| cbdca |
| MFCD00070464 |
| (SP-4-2)-diammine(1,1-cyclobutanedicarboxylato)platinum(II) |
| [Cyclobutane-1,1-dicarboxylato(2-)-κO,O]platinum diammoniate |
| cis-diammine(1,1-cyclobutanedicarboxylato)platinum(II) |
| [1,1-Cyclobutanedicarboxylato(2-)-κO,O]platinum diammoniate |
| Platinum, [1,1-cyclobutanedicarboxylato(2-)-κO,κO]-, ammoniate (1:2) |
| JM-8 |
| PARAPLATIN |
| cis-diammine(cyclobutane-1,1-dicarboxylato)platinum(II) |
| PARAPLATIN(R) |
| CARBONPLATIN |
| cis-Diamine(1,1-cyclobutanedicarboxylato)platinum(II) |
| CARBOPLATINUM |
| diammine[cyclobutane-1,1-dicarboxylato(2-)-κ2O1,O1]platinum |
| cis-diammmine(1,1-cyclobutanedicarboxylato)platinum(II) |
| Platinum, diammine[1,1-cyclobutanedicarboxylato(2-)-κO,κO]- |
| EINECS 255-446-0 |
| Carboplatin |
| diammine(cyclobutane-1,1-dicarboxylato)platinum(II) |
| platinum, diammine[1,1-cyclobutanedicarboxylato(2-)-κO1,κO1]- |
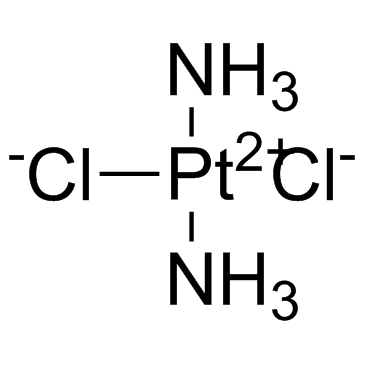

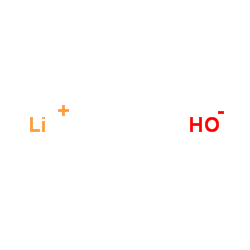
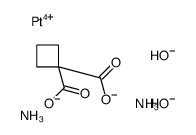 CAS#:113287-15-3
CAS#:113287-15-3 CAS#:15730-38-8
CAS#:15730-38-8
