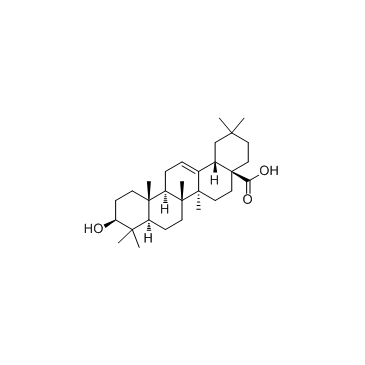
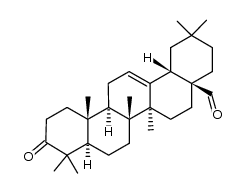
3-oxo-Olean-12-en-28-oic acid
3-oxo-Olean-12-en-28-oic acid structure
|
Common Name | 3-oxo-Olean-12-en-28-oic acid | ||
|---|---|---|---|---|
| CAS Number | 17990-42-0 | Molecular Weight | 454.684 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 551.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C30H46O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 301.5±26.6 °C | |
Use of 3-oxo-Olean-12-en-28-oic acidOleanolic acid is a triterpenoid, inhibits infection by HIV-1 in in vitro infected PBMC, naturally infected PBMC and monocyte/macrophages with EC50 of 22.7 mM, 24.6 mM and 57.4 mM, respectively. Besides,it has IC50 of 17μM for the production of leukotriene B4 from rat peritoneal leukocytes.IC50:17μM(The production of leukotriene B4 from rat peritoneal leukocytes)[1]IC50:22.7 mM, 24.6 mM and 57.4 mM(in vitro infected PBMC, naturally infected PBMC and monocyte/macrophages by HIV-1, respectively.[2]In vitro: The highest of the four tested doses (100 μM), showed only a slight inhibition approximately, 30%. In contrast, the more powerful effect of oleanonic acid in this system, suggests that it acts through a mechanism related to the inhibition of 5-lipoxygenase, either directly or interfering with some of the mechanisms that participate in the complex activation of this enzyme. Oleanonic acid also acts by reducing prostaglandin synthesis.[1]Oleanolic acid inhibits the HIV-1 replication in all the cellular systems used (EC50 values: 22.7 microM, 24.6 microM and 57.4 microM for in vitro infected PBMC, naturally infected PBMC and M/M, respectively). As regards the mechanism of action, oleanolic acid inhibits in vitro the HIV-1 protease activity.[2]In vivo: Oleanonic acid exerted no activity on the oedema induced by application of ethyl phenylpropiolate after a pre-treatment of 16 h. In the TPA ear oedema test, it showed a non-significant 28% inhibition. However, when assayed on the ear oedema induced by DPP, oleanonic acid reduced the swelling by 40%, an effect similar to that of the standard carbamazepine. In the mouse model of delayed hypersensitivity induced by dinitrofluorobenzene, oleanonic acid was ineffective at both 24 and 96 h, while oleanolic acid reduced non-significantly the oedema at 96 h by 32%.In the TPA model of chronic inflammation induced by multiple applications, oleanonic acid showed a significant effect, with 45% inhibition. In contrast, oleanolic acid was inactive. Both inhibited the neutrophil infiltration measured as myeloperoxidase activity by 84% and 67%, respectively. The inhibition observed for dexamethasone on the swelling and myeloperoxidase activity was around 90%. The histological study of ears treated only with repeated doses of TPA showed an extensive diffusive inflammatory lesion with microabscesses affecting dermis and epidermis. The main infiltrating cells in the skin were neutrophils and epithelial thickness was 6.6±1.0 cells. In the tissues treated only with the solvent acetone, epithelial thickness was 2.1±0.5 and no signs of lesion or leukocyte infiltration were detectable. The multidose treatment with oleanonic acid reduced both the intensity and extension of the damage produced by TPA, as this was localized in the dermis, where the main infiltrating cells were lymphocytes, and where fibrosis was observed. In this case, epithelium thickness was 4.4±0.7 cells. The ears treated with dexamethasone showed minimal inflammatory lesions and sometimes none at all, and the epithelium thickness was 4.3±0.7 cells.The paw oedema induced by bradykinin was significantly reduced (61%) by oleanonic acid, whereas isoprenaline had a slightly lower effect (52%). Both oleanolic and oleanonic acid also reduced the paw oedema induced by phospholipase A2; the latter showing its strongest effect at 60 min, with an 84% inhibition, and maintaining activity at 90 min. Oleanolic acid also had its maximum effect at 60 min, vanishing at 90 min, while the activity of cyproheptadine was uniform along the experiment, ranging 80–90% inhibition .[1] |
| Name | (4aS,6aR,6aS,6bR,8aR,12aR,14bS)-2,2,6a,6b,9,9,12a-heptamethyl-10-oxo-3,4,5,6,6a,7,8,8a,11,12,13,14b-dodecahydro-1H-picene-4a-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Oleanolic acid is a triterpenoid, inhibits infection by HIV-1 in in vitro infected PBMC, naturally infected PBMC and monocyte/macrophages with EC50 of 22.7 mM, 24.6 mM and 57.4 mM, respectively. Besides,it has IC50 of 17μM for the production of leukotriene B4 from rat peritoneal leukocytes.IC50:17μM(The production of leukotriene B4 from rat peritoneal leukocytes)[1]IC50:22.7 mM, 24.6 mM and 57.4 mM(in vitro infected PBMC, naturally infected PBMC and monocyte/macrophages by HIV-1, respectively.[2]In vitro: The highest of the four tested doses (100 μM), showed only a slight inhibition approximately, 30%. In contrast, the more powerful effect of oleanonic acid in this system, suggests that it acts through a mechanism related to the inhibition of 5-lipoxygenase, either directly or interfering with some of the mechanisms that participate in the complex activation of this enzyme. Oleanonic acid also acts by reducing prostaglandin synthesis.[1]Oleanolic acid inhibits the HIV-1 replication in all the cellular systems used (EC50 values: 22.7 microM, 24.6 microM and 57.4 microM for in vitro infected PBMC, naturally infected PBMC and M/M, respectively). As regards the mechanism of action, oleanolic acid inhibits in vitro the HIV-1 protease activity.[2]In vivo: Oleanonic acid exerted no activity on the oedema induced by application of ethyl phenylpropiolate after a pre-treatment of 16 h. In the TPA ear oedema test, it showed a non-significant 28% inhibition. However, when assayed on the ear oedema induced by DPP, oleanonic acid reduced the swelling by 40%, an effect similar to that of the standard carbamazepine. In the mouse model of delayed hypersensitivity induced by dinitrofluorobenzene, oleanonic acid was ineffective at both 24 and 96 h, while oleanolic acid reduced non-significantly the oedema at 96 h by 32%.In the TPA model of chronic inflammation induced by multiple applications, oleanonic acid showed a significant effect, with 45% inhibition. In contrast, oleanolic acid was inactive. Both inhibited the neutrophil infiltration measured as myeloperoxidase activity by 84% and 67%, respectively. The inhibition observed for dexamethasone on the swelling and myeloperoxidase activity was around 90%. The histological study of ears treated only with repeated doses of TPA showed an extensive diffusive inflammatory lesion with microabscesses affecting dermis and epidermis. The main infiltrating cells in the skin were neutrophils and epithelial thickness was 6.6±1.0 cells. In the tissues treated only with the solvent acetone, epithelial thickness was 2.1±0.5 and no signs of lesion or leukocyte infiltration were detectable. The multidose treatment with oleanonic acid reduced both the intensity and extension of the damage produced by TPA, as this was localized in the dermis, where the main infiltrating cells were lymphocytes, and where fibrosis was observed. In this case, epithelium thickness was 4.4±0.7 cells. The ears treated with dexamethasone showed minimal inflammatory lesions and sometimes none at all, and the epithelium thickness was 4.3±0.7 cells.The paw oedema induced by bradykinin was significantly reduced (61%) by oleanonic acid, whereas isoprenaline had a slightly lower effect (52%). Both oleanolic and oleanonic acid also reduced the paw oedema induced by phospholipase A2; the latter showing its strongest effect at 60 min, with an 84% inhibition, and maintaining activity at 90 min. Oleanolic acid also had its maximum effect at 60 min, vanishing at 90 min, while the activity of cyproheptadine was uniform along the experiment, ranging 80–90% inhibition .[1] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 551.7±50.0 °C at 760 mmHg |
| Molecular Formula | C30H46O3 |
| Molecular Weight | 454.684 |
| Flash Point | 301.5±26.6 °C |
| Exact Mass | 454.344696 |
| PSA | 54.37000 |
| LogP | 8.48 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | -20°C |
|
~10% 
3-oxo-Olean-12-... CAS#:17990-42-0 |
| Literature: Organic and Biomolecular Chemistry, , vol. 11, # 10 p. 1726 - 1738 |
|
~93% 
3-oxo-Olean-12-... CAS#:17990-42-0 |
| Literature: Molecules, , vol. 18, # 3 p. 3615 - 3629 |
|
~% 
3-oxo-Olean-12-... CAS#:17990-42-0 |
| Literature: Gazzetta Chimica Italiana, , vol. 104, p. 1269 - 1278 |
|
~% 
3-oxo-Olean-12-... CAS#:17990-42-0 |
| Literature: Pharmazie, , vol. 42, # 1 p. 67 |
|
~% 
3-oxo-Olean-12-... CAS#:17990-42-0 |
| Literature: Journal of the Chemical Society, , vol. 103, p. 2050,2057 Justus Liebigs Annalen der Chemie, , vol. 517, p. 211,222 Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, , vol. 215, p. 199,202 Berichte der Deutschen Pharmazeutischen Gesellschaft, , vol. 15, p. 121 Chem. Zentralbl., , vol. 76, # II p. 533 Yakugaku Zasshi, , vol. 54, p. 67 Chem. Zentralbl., , vol. 105, # II p. 2536 Acta Phytochimica, , vol. 6, p. 211 Chem. Zentralbl., , vol. 103, # II p. 1790 Collection of Czechoslovak Chemical Communications, vol. 5, p. 165 Chem. Zentralbl., , vol. 104, # II p. 1035 Collection of Czechoslovak Chemical Communications, vol. 2, p. 414 Chem. Zentralbl., , vol. 101, # II p. 3292 Acta Phytochimica, , vol. 7, p. 180 Chem. Zentralbl., , vol. 105, # I p. 2766 Chemicke Listy, , vol. 25, p. 393,396 Chem. Zentralbl., , vol. 103, # I p. 1247 |
|
~% 
3-oxo-Olean-12-... CAS#:17990-42-0 |
| Literature: Helvetica Chimica Acta, , vol. 21, p. 1371,1382 Acta Phytochimica, , vol. 7, p. 180 Chem. Zentralbl., , vol. 105, # I p. 2766 |
|
~% 
3-oxo-Olean-12-... CAS#:17990-42-0 |
| Literature: Helvetica Chimica Acta, , vol. 19, p. 247,249 Recueil des Travaux Chimiques des Pays-Bas, , vol. 51, p. 1200,1202 |
| oleanoic acid |
| 3-oxooleana-12-en-28-oic acid |
| Olean-12-en-28-oic acid,3-oxo |
| oleanonic acid |
| 3-oxo-oleanolic acid |
| 3-Oxoolean-12-en-28-oic acid |
| Olean-12-en-28-oic acid, 3-oxo- |
| 3-keto oleanolic acid |
| 3-oxo-olean-12-en-28-oic acid |