BAY 11-7082
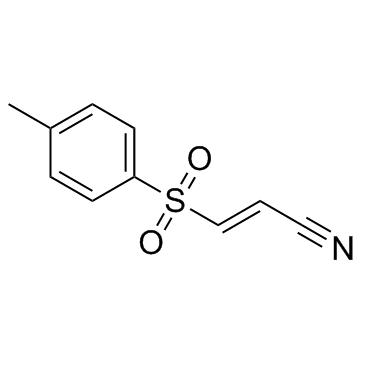
BAY 11-7082 structure
|
Common Name | BAY 11-7082 | ||
|---|---|---|---|---|
| CAS Number | 19542-67-7 | Molecular Weight | 207.249 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 397.6±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H9NO2S | Melting Point | 133-135℃ | |
| MSDS | Chinese USA | Flash Point | 194.3±27.9 °C | |
Use of BAY 11-7082BAY 11-7082 is a NF-κB inhibitor which decreases NF-κB by inhibiting TNF-α-induced phosphorylation of IκB-α. BAY 11-7082 inhibits ubiquitin-specific protease USP7 and USP21 with IC50s of 0.19 μM and 0.96 μM, respectively. |
| Name | (E)-3-tosylacrylonitrile |
|---|---|
| Synonym | More Synonyms |
| Description | BAY 11-7082 is a NF-κB inhibitor which decreases NF-κB by inhibiting TNF-α-induced phosphorylation of IκB-α. BAY 11-7082 inhibits ubiquitin-specific protease USP7 and USP21 with IC50s of 0.19 μM and 0.96 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
NF-κB USP7:0.19 μM (IC50) USP21:0.96 μM (IC50) Autophagy |
| In Vitro | Bay 11-7082, an inhibitor of NF-κB, induces apoptosis of HTLV-I-infected T-cell lines but only negligible apoptosis of HTLV-I-negative T cells. Bay 11-7082 rapidly and efficiently reduces the DNA binding of NF-κB in HTLV-I-infected T-cell lines and down-regulated the expression of the antiapoptotic gene, Bcl-xL, regulated by NF-κB. Bay 11-7082 selectively inhibits Tax-induced NF-κB activity in a human T-cell line[1]. BAY 11-7082 inhibits NFκB signalling and is recently shown to inhibit the majority of E2 and E3 ligases tested by reacting covalently with the catalytic cysteine residues. Moreover, BAY 11-7082 also inhibits several tyrosine phosphatases by reacting with catalytic Cys residue of these enzymes. NSC 697923 is originally shown to inhibit the E2 ligase Ubc13-Uev1A[2]. BAY 11-7082 inhibits the phosphorylation of IκBα and activation of NF-κB, induces the death of HBL-1 cells. BAY 11-7082 completely suppresses the LPS-stimulated and IL-1-stimulated phosphorylation of the activation loop of IKKβ[3]. BAY 11-7082 acts by inhibiting TNF-α-induced phosphorylation of IκB-α, resulting in decreased NF-κB and decreases expression of adhesion molecules[4]. |
| Kinase Assay | UBE1 (0.17 μM) in 22.5 μL of 20 mM Hepes, pH 7.5, containing 10 μM ubiquitin is incubated for 45 min at 21°C with 1 μL of DMSO or 1 μL of BAY 11-7082 in DMSO. A 2.5 μL solution of 10 mM magnesium acetate and 0.2 mM ATP is added, incubated for 10 min at 30°C, and the reactions are terminated by the addition of 2.5 μL of 10% (w/v) SDS and heating for 6 min at 75°C. The samples are subjected to SDS/PAGE in the absence of any thiol. The gels are stained for 1 h with Coomassie Instant Blue and destained by washing with water. The loading of ubiquitin to E2 conjugating enzymes is carried out in an identical manner, except that UBE1 (0.17 μM) is mixed with Ubc13 (2.4 μM) or UbcH7 (2.9 μM) prior to incubation with BAY 11-7082[3]. |
| Cell Assay | The effect of Bay 11-7082 on cell growth is assayed by the WST-1 method. 2×104 (cell lines) or 2×105 (PBMCs) cells are incubated in a 96-well microculture plate under the above conditions in the absence or presence of various concentrations of Bay 11-7082 (1, 2, 3, 4, and 5 μM). After 48 hours of culture, 10 μL WST-1 solution is added and the cells are further incubated for another 2 hours. The number of surviving cells is measured with a microplate reader at a reference wavelength of 655 nm and test wavelength of 450 nm. Cell viability is determined as percentage of the control (ie, absence of Bay 11-7082)[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 397.6±42.0 °C at 760 mmHg |
| Melting Point | 133-135℃ |
| Molecular Formula | C10H9NO2S |
| Molecular Weight | 207.249 |
| Flash Point | 194.3±27.9 °C |
| Exact Mass | 207.035400 |
| PSA | 66.31000 |
| LogP | 1.28 |
| Appearance of Characters | solid | white |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.557 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: 25 mg/mL, soluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UD1430000 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
|
Inhibition of STAT5a by Naa10p contributes to decreased breast cancer metastasis.
Carcinogenesis 35(10) , 2244-53, (2014) N-α-Acetyltransferase 10 protein (Naa10p, also called arrest-defective 1), the catalytic subunit of N-acetyltransferase A, is a critical regulator of cell death and proliferation. Naa10p is also shown... |
|
|
Extra-Nuclear Signaling Pathway Involved in Progesterone-Induced Up-Regulations of p21cip1 and p27kip1 in Male Rat Aortic Smooth Muscle Cells.
PLoS ONE 10 , e0125903, (2015) Previously, we demonstrated that progesterone (P4) at physiologic levels (5-500 nM) inhibited proliferation in cultured rat aortic smooth muscle cells (RASMCs) through a P4 receptor (PR)-dependent pat... |
|
|
TRIB3 mediates the expression of Wnt5a and activation of nuclear factor-κB in Porphyromonas endodontalis lipopolysaccharide-treated osteoblasts.
Mol. Oral Microbiol. 30 , 295-306, (2015) Porphyromonas endodontalis lipopolysaccharide (LPS) is considered to be correlated with the progression of bone resorption in periodontal and periapical diseases. Wnt5a has recently been implicated in... |
| 3-(p-Tolylsulfonyl)acrylonitrile |
| 3-(p-Toluenesulfonyl)acrylonitrile |
| (E)-3-Tosylacrylonitrile |
| (2E)-3-[(4-Methylphenyl)sulfonyl]acrylonitrile |
| (E)-3-(4-Methylphenylsulfonyl)-2-propenenitrile |
| (E)-3-(4-methylphenyl)sulfonylprop-2-enenitrile |
| 3-((4-Methylphenyl)sulfonyl)-2-propenenitrile |
| BAY-11-7082 |
| 2-Propenenitrile, 3-((4-methylphenyl)sulfonyl)- |
| BAY 11-7082 |
| (2E)-3-[(4-methylphenyl)sulfonyl]prop-2-enenitrile |
| 2-Propenenitrile, 3-[(4-methylphenyl)sulfonyl]-, (2E)- |
| (E)-3-(p-Toluenesulfonyl)acrylonitrile |
| BAY11-7082 |
 CAS#:824-79-3
CAS#:824-79-3 CAS#:107-13-1
CAS#:107-13-1 CAS#:77825-72-0
CAS#:77825-72-0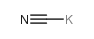 CAS#:151-50-8
CAS#:151-50-8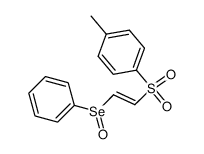 CAS#:86409-95-2
CAS#:86409-95-2 CAS#:86409-89-4
CAS#:86409-89-4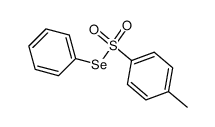 CAS#:68819-94-3
CAS#:68819-94-3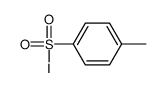 CAS#:1950-78-3
CAS#:1950-78-3 CAS#:4554-16-9
CAS#:4554-16-9 CAS#:1950-69-2
CAS#:1950-69-2 CAS#:715-12-8
CAS#:715-12-8
