O6-Benzylguanine
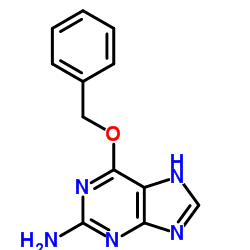
O6-Benzylguanine structure
|
Common Name | O6-Benzylguanine | ||
|---|---|---|---|---|
| CAS Number | 19916-73-5 | Molecular Weight | 241.249 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 621.4±63.0 °C at 760 mmHg | |
| Molecular Formula | C12H11N5O | Melting Point | 193(dec.) | |
| MSDS | Chinese USA | Flash Point | 329.6±33.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of O6-BenzylguanineO6-Benzylguanine, a guanine analog, is the DNA repair enzyme O6-alkylguanine-DNA alkyltransferase (MGMT/AGT) inhibitor. O6-Benzylguanine acts as an AGT substrate, which transfers its benzyl group to the AGT cysteine residue, thereby irreversibly inactivating AGT and preventing DNA repair. O6-Benzylguanine induces tumor cell apoptosis. Antineoplastic activity[1][2]. |
| Name | 6-phenylmethoxy-7H-purin-2-amine |
|---|---|
| Synonym | More Synonyms |
| Description | O6-Benzylguanine, a guanine analog, is the DNA repair enzyme O6-alkylguanine-DNA alkyltransferase (MGMT/AGT) inhibitor. O6-Benzylguanine acts as an AGT substrate, which transfers its benzyl group to the AGT cysteine residue, thereby irreversibly inactivating AGT and preventing DNA repair. O6-Benzylguanine induces tumor cell apoptosis. Antineoplastic activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | The L3.6pl cells are relatively sensitive to O6-Benzylguanine (24-72 hours) in a dose- and time-dependent manner. The IC50 is 50 μg (at 48 hours)[2]. O6-Benzylguanine (50 μg; 48 hours) modulates p53 downstream target protein expression, induces apoptosis, and decreases cell proliferation[2]. O6-Benzylguanine (50 μg; 48 hours) significantly decreases the MGMT transcriptional activity in L3.6pl[2]. Western Blot Analysis[2] Cell Line: L3.6pl and PANC1 cells Concentration: 50 μg Incubation Time: 48 hours Result: Expressions of O6 methyl guanine DNA methyl transferase (MGMT), cyclin B1, cyclin B2, cyclin A, p53, and ki-67 were decreased, whereas p21 was increased. The levels of cyto C and caspase 9 were increased, whereas the levels of PARP1 protein were decreased. RT-PCR[2] Cell Line: L3.6pl cells Concentration: 50 μg Incubation Time: 48 hours Result: Decreased the MGMT transcriptional activity in L3.6pl. |
| In Vivo | O6-Benzylguanine (100 μg; i.p.; daily for 35 days) inhibits pancreatic cancer cell growth and increases pancreatic cell sensitivity to Gemcitabine (100 mg/kg)[2]. O6-Benzylguanine inhibits pancreatic cancer cell proliferation and induces tumor cell apoptosis in vivo[2]. Animal Model: Male athymic nude mice (NCI-nu) (bearing human pancreatic cancer L3.6pl cells)[2] Dosage: 100 μg Administration: i.p; daily for 35 days Result: Significantly decreased median tumor volume and weight. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 621.4±63.0 °C at 760 mmHg |
| Melting Point | 193(dec.) |
| Molecular Formula | C12H11N5O |
| Molecular Weight | 241.249 |
| Flash Point | 329.6±33.7 °C |
| Exact Mass | 241.096359 |
| PSA | 89.71000 |
| LogP | 1.95 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.743 |
| Storage condition | -20°C Freezer |
| Water Solubility | methanol: 20 mg/mL |
|
Material Safety Data Sheet
Section1. Identification of the substance O6-Benzylguanine Product Name: Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed.
H315:Causes skin irritation H319:Causes serious eye irritation H335:May cause respiratory irritation P261:Avoid breathing dust/fume/gas/mist/vapours/spray Wear protective gloves/protective clothing/eye protection/face protection P280: P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing P304+P340:IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing P405:Store locked up Section3. Composition/information on ingredients. O6-Benzylguanine Ingredient name: CAS number:19916-73-5 Section4. First aid measures Immediately wash skin with copious amounts of water for at least 15 minutes while removing Skin contact: contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Ingestion: Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Storage:Store in closed vessels. Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Not specified Appearance: Boiling point:No data Melting point:No data Flash point:No data Density:No data Molecular formula:C12H11N5O Molecular weight:241.3 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 5 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Differential expression of miR200a-3p and miR21 in grade II-III and grade IV gliomas: evidence that miR200a-3p is regulated by O⁶-methylguanine methyltransferase and promotes temozolomide responsiveness.
Cancer Biol. Ther. 15(7) , 938-50, (2014) Glioblastoma multiforme (GBM) is the most common primary brain tumor and is among the deadliest of human cancers. Dysregulation of microRNAs (miRNAs) expression is an important step in tumor progressi... |
|
|
Formation and repair of pyridyloxobutyl DNA adducts and their relationship to tumor yield in A/J mice.
Chem. Res. Toxicol. 25(10) , 2167-78, (2012) The nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a known human carcinogen. It generates methyl and pyridyloxobutyl DNA adducts. The role of the methyl DNA adducts has been well-... |
|
|
Evaluation of novel imidazotetrazine analogues designed to overcome temozolomide resistance and glioblastoma regrowth.
Mol. Cancer Ther. 14(1) , 111-9, (2015) The cellular responses to two new temozolomide (TMZ) analogues, DP68 and DP86, acting against glioblastoma multiforme (GBM) cell lines and primary culture models are reported. Dose-response analysis o... |
| O(6)-Benzylguanine |
| 7H-purin-2-amine, 6-(phenylmethoxy)- |
| O6-Benzylguanine |
| 2-amino-6-(phenylmethoxy)-9H-purine |
| O(6)-bGua |
| 2-Amino-6-benzyloxy-9H-purine |
| O-6-Benzylguanine |
| 6-Benzyloxy guanine |
| 6-(Benzyloxy)-3H-purin-2-amine |
| 2-Amino-6-(benzyloxy)purine |
| 6-O-Benzylguanine |
| 9H-Purin-2-amine, 6-(phenylmethoxy)- |
| 6-(benzyloxy)guanine |
| O-Benzylguanine |
| 6-(phenylmethoxy)-9H-purin-2-amine |
| MFCD00269931 |
| amino-6-(phenylmethoxy)-9H-purine |
| 6-(Benzyloxy)-7H-purin-2-amine |
 CAS#:10310-21-1
CAS#:10310-21-1 CAS#:100-51-6
CAS#:100-51-6 CAS#:612507-56-9
CAS#:612507-56-9 CAS#:34798-95-3
CAS#:34798-95-3 CAS#:20194-18-7
CAS#:20194-18-7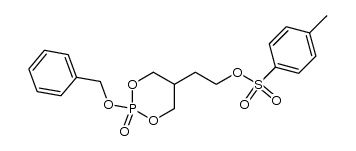 CAS#:100199-49-3
CAS#:100199-49-3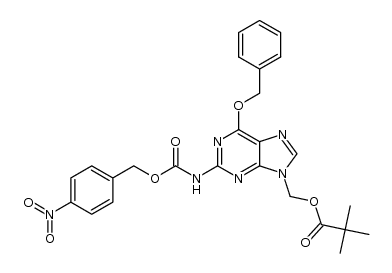 CAS#:1340543-34-1
CAS#:1340543-34-1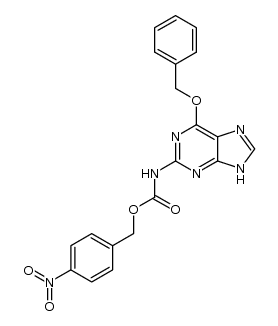 CAS#:1340543-31-8
CAS#:1340543-31-8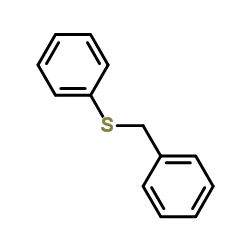 CAS#:831-91-4
CAS#:831-91-4 CAS#:73-40-5
CAS#:73-40-5 CAS#:142217-77-4
CAS#:142217-77-4 CAS#:86357-09-7
CAS#:86357-09-7 CAS#:92193-74-3
CAS#:92193-74-3
