MRS 1523
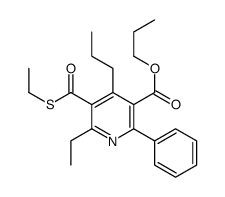
MRS 1523 structure
|
Common Name | MRS 1523 | ||
|---|---|---|---|---|
| CAS Number | 212329-37-8 | Molecular Weight | 399.54600 | |
| Density | 1.1g/cm3 | Boiling Point | 551.3ºC at 760 mmHg | |
| Molecular Formula | C23H29NO3S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 287.2ºC | |
Use of MRS 1523MRS 1523 is a potent and selective adenosine A3 receptor antagonist with Ki values of 18.9 nM and 113 nM for human and rat A3 receptors, respectively. In rat this corresponds to selectivities of 140- and 18-fold vs A1 and A2A receptors, respectively. MRS 1523 can exert antihyperalgesic effect through N-type Ca channel block and action potential inhibition in isolated rat dorsal root ganglion (DRG) neurons[1][2]. |
| Name | propyl 6-ethyl-5-ethylsulfanylcarbonyl-2-phenyl-4-propylpyridine-3-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | MRS 1523 is a potent and selective adenosine A3 receptor antagonist with Ki values of 18.9 nM and 113 nM for human and rat A3 receptors, respectively. In rat this corresponds to selectivities of 140- and 18-fold vs A1 and A2A receptors, respectively. MRS 1523 can exert antihyperalgesic effect through N-type Ca channel block and action potential inhibition in isolated rat dorsal root ganglion (DRG) neurons[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 18.9 nM (Human A3 receptor), 113 nM (Rat A3 receptor), 15.6 µM (A1 receptor) and 2.05 µM (A2A receptor)[1] |
| In Vitro | MRS 1523 (0.1-1 μM) treatment significantly antagonizes cell numbers to 40.7% and 57.4% of the control values, respectively, 30 min before the addition of cordycepin (60 μM). MRS1523 (1 μM) alone has any effect on tumor cell growth[3]. A partial blockade of the adenosine-5'-N-ethylcarboxamide (NECA)-induced migration is observed when human endothelial progenitor cells (hEPC) are co-incubated with MRS 1523 (1 nM). Furthermore, in 3-days hEPC, the treatment with MRS 1523 100 nM inhibits the NECA-induced migration by 70%. NECA-induced migration is blocked in dose-response fashion by MRS 1523 with calculated Ki of 147 nM[4]. Cell Viability Assay[3] Cell Line: B16-BL6 cells Concentration: 0.1 µM, 1 µM Incubation Time: 24 hours, 48 hours, 72 hours Result: Antagonized the growth suppression induced by cordycepin. |
| In Vivo | The expression and functional effects of A3 adenosine receptor (A3AR) on the excitability of small- to medium-sized, capsaicin-sensitive, dorsal root ganglion (DRG) neurons isolated from 3- to 4-week-old rats are investigated. The endogenous agonist adenosine reduces N-type Ca currents, and its effect is inhibited by 56% in the presence of A3AR antagonist MRS 1523. Current-clamp recordings demonstrated that neuronal firing of rat DRG neurons was also significantly reduced by A3AR activation in a MRS 1523-sensitive but PD173212-insensitive manner[2]. |
| References |
| Density | 1.1g/cm3 |
|---|---|
| Boiling Point | 551.3ºC at 760 mmHg |
| Molecular Formula | C23H29NO3S |
| Molecular Weight | 399.54600 |
| Flash Point | 287.2ºC |
| Exact Mass | 399.18700 |
| PSA | 81.56000 |
| LogP | 5.72360 |
| Index of Refraction | 1.554 |
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate.
J. Neurosci. 30 , 10282-93, (2010) Prostatic acid phosphatase (PAP) is expressed in nociceptive dorsal root ganglion (DRG) neurons, functions as an ectonucleotidase, and generates adenosine extracellularly. Here, we found that PAP inhi... |
|
|
Structure-activity relationships and molecular modeling of 3, 5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists.
J. Med. Chem. 41 , 3186, (1998) The structure-activity relationships of 6-phenyl-1,4-dihydropyridine derivatives as selective antagonists at human A3 adenosine receptors have been explored (Jiang et al. J. Med. Chem. 1997, 39, 4667-... |
|
|
A3 adenosine receptors regulate Cl- channels of nonpigmented ciliary epithelial cells.
Am. J. Physiol. 276 , C659, (1999) Adenosine stimulates Cl- channels of the nonpigmented (NPE) cells of the ciliary epithelium. We sought to identify the specific adenosine receptors mediating this action. Cl- channel activity in immor... |
| MRS 1523 |
| Lopac-M-1809 |
| 3-Propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate |