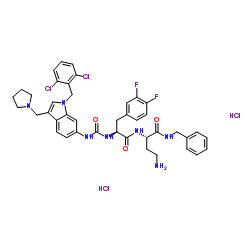
| Description |
RWJ-56110 is a potent, selective, peptide-mimetic inhibitor of PAR-1 activation and internalization (binding IC50=0.44 uM) and shows no effect on PAR-2, PAR-3, or PAR-4. RWJ-56110 inhibits the aggregation of human platelets induced by both SFLLRN-NH2 (IC50=0.16 μM) and thrombin (IC50=0.34 μM), quite selective relative to U46619 (HY-108566). RWJ-56110 blocks angiogenesis and blocks the formation of new vessel in vivo. RWJ-56110 induces cell apoptosis[1][2].
|
| Related Catalog |
|
| In Vitro |
Proteinase-activated receptors (PARs) are a family of G protein-coupled receptors activated by the proteolytic cleavage of their N-terminal extracellular domain, exposing a new amino terminal sequence that functions as a tethered ligand to activate the receptors. RWJ56110 inhibits the aggregation of human platelets induced by both SFLLRN-NH2 (IC50=0.16 μM) and thrombin (IC50=0.34 μM) while being quite selective relative to collagen and the thromboxane mimetic U46619 (HY-108566)[1]. RWJ-56110 is fully inhibits thrombin-induced RASMC proliferation with an IC50 value of 3.5 μM. RWJ-56110 shows blockade of thrombin’s action with RASMC calcium mobilization (IC50=0.12 μM), as well as with HMVEC (IC50=0.13 μM) and HASMC calcium mobilization (IC50=0.17 μM)[1]. RWJ56110 (0.1-10 μM; 24-96 hours) inhibits endothelial cell growth dose-dependently, with half-maximal inhibitory concentration of RWJ56110 is approximately 10 μM[2]. RWJ56110 (0.1-10 μM; 6 hours) inhibits DNA synthesis of endothelial cells in a thymidine incorporation assays. Endothelial cells are in fast-growing state (50-60% confluence), RWJ56110 inhibits cell DNA synthesis in a dose-dependent manner, but when cells that are in the quiescent state (100% confluent), the inhibitory effect of PAR-1 antagonists is much less pronounced[2]. RWJ56110 (0.1-10 μM; pretreatment for 15 min) inhibits thrombin-induced Erk1/2 activation in a concentration-dependent manner. However, when endothelial cells are stimulated by FBS (final concentration 4%), it reduces partially the activated levels of Erk1/2[2]. RWJ56110 (30 μM; 24 hours) has an inhibitory effect on endothelial cell cycle progression. It reduces the percentage of cells in the S phase, while alterations in the percentages of G1 and G2/M cells are less pronounced[2]. Western Blot Analysis[2] Cell Line: Endothelial cells Concentration: 0 μM; 3 μM; 1 μM; 3 μM; 10 μM Incubation Time: Pretreatment for 15 min Result: Resuted in MAPK activation in Endothelial cells. Cell Cycle Analysis[2] Cell Line: Endothelial cells Concentration: 0 μM; 3 μM; 1 μM; 3 μM; 10 μM Incubation Time: Pretreatment for 15 min Result: Reduced cell number in S phase.
|
| References |
[1]. Andrade-Gordon, et al.Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tethered-ligand receptor. oc Natl Acad Sci U S A. 1999 Oct 26;96(22):12257-62. [2]. Panagiota Zania, et al. Blockade of angiogenesis by small molecule antagonists to protease-activated receptor-1: association with endothelial cell growth suppression and induction of apoptosis. J Pharmacol Exp Ther. 2006 Jul;318(1):246-54.
|