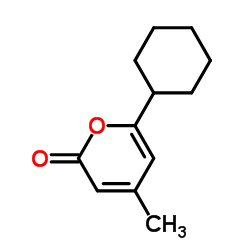
Ciclopirox
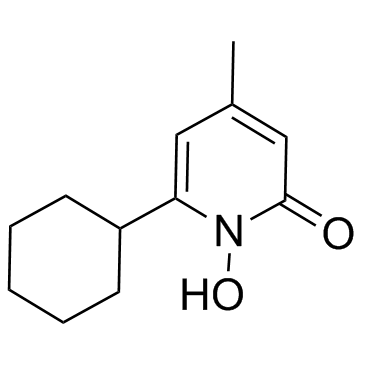
Ciclopirox structure
|
Common Name | Ciclopirox | ||
|---|---|---|---|---|
| CAS Number | 29342-05-0 | Molecular Weight | 207.269 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 350.0±25.0 °C at 760 mmHg | |
| Molecular Formula | C12H17NO2 | Melting Point | 1440C | |
| MSDS | N/A | Flash Point | 165.5±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CiclopiroxCiclopirox (Penlac) is a synthetic antifungal agent.Target: AntifungalCiclopirox is a synthetic antifungal agent for topical dermatologic treatment of superficial mycoses. It is most useful against Tinea versicolor. The mechanism of action of ciclopirox is poorly understood [1]. However, loss of function of certain catalase and peroxidase enzymes has been implicated as the mechanism of action, as well as various other components of cellular metabolism. In a study conducted to further elucidate ciclopirox's mechanism, several Saccharomyces cerevisiae mutants were screened and tested. Results from interpretation of the effects of both the drug treatment and mutation suggested that ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport [2]. It acts by inhibiting the membrane transfer system by interrupting the Na+ K+ ATPase [1]. It is currently being investigated as an alternative treatment to ketoconazole for seborrhoeic dermatitis as it suppresses growth of the yeast Malassezia furfur. Initial results show similar efficacy to ketoconazole with a relative increase in subjective symptom relief due to its inherent anti-inflammatory properties [3]. |
| Name | ciclopirox |
|---|---|
| Synonym | More Synonyms |
| Description | Ciclopirox (Penlac) is a synthetic antifungal agent.Target: AntifungalCiclopirox is a synthetic antifungal agent for topical dermatologic treatment of superficial mycoses. It is most useful against Tinea versicolor. The mechanism of action of ciclopirox is poorly understood [1]. However, loss of function of certain catalase and peroxidase enzymes has been implicated as the mechanism of action, as well as various other components of cellular metabolism. In a study conducted to further elucidate ciclopirox's mechanism, several Saccharomyces cerevisiae mutants were screened and tested. Results from interpretation of the effects of both the drug treatment and mutation suggested that ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport [2]. It acts by inhibiting the membrane transfer system by interrupting the Na+ K+ ATPase [1]. It is currently being investigated as an alternative treatment to ketoconazole for seborrhoeic dermatitis as it suppresses growth of the yeast Malassezia furfur. Initial results show similar efficacy to ketoconazole with a relative increase in subjective symptom relief due to its inherent anti-inflammatory properties [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 350.0±25.0 °C at 760 mmHg |
| Melting Point | 1440C |
| Molecular Formula | C12H17NO2 |
| Molecular Weight | 207.269 |
| Flash Point | 165.5±23.2 °C |
| Exact Mass | 207.125931 |
| PSA | 42.23000 |
| LogP | 2.59 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Storage condition | -20°C Freezer |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | C,O |
| Risk Phrases | R8:Contact with combustible material may cause fire. R35:Causes severe burns. R34:Causes burns. R20:Harmful by inhalation. |
| Safety Phrases | S23-S26-S36-S45 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 1 |
| RTECS | QU5900000 |
| Packaging Group | II |
| Hazard Class | 8 |
| HS Code | 2933790090 |
|
~% 
Ciclopirox CAS#:29342-05-0 |
| Literature: Hoechst Aktiengesellschaft Patent: US3972888 A1, 1976 ; |
|
~% 
Ciclopirox CAS#:29342-05-0 |
| Literature: Hoechst Aktiengesellschaft Patent: US3972888 A1, 1976 ; |
|
~% 
Ciclopirox CAS#:29342-05-0 |
| Literature: Hoechst Aktiengesellschaft Patent: US3972888 A1, 1976 ; |
|
~% 
Ciclopirox CAS#:29342-05-0 |
| Literature: Lohaus; Dittmar Arzneimittel-Forschung/Drug Research, 1981 , vol. 31, # 8 a p. 1311 - 1316 |
|
~% 
Ciclopirox CAS#:29342-05-0 |
| Literature: Lohaus; Dittmar Arzneimittel-Forschung/Drug Research, 1981 , vol. 31, # 8 a p. 1311 - 1316 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Effect of Wnt inhibitors in pancreatic cancer.
Anticancer Res. 34(10) , 5375-80, (2014) Activated Wnt signaling in cancer cells leads to cell proliferation. It has been shown that the Wnt pathway is activated in pancreatic adenocarcinoma cells. Therefore, we tested the effect of Wnt inhi... |
|
|
The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment.
Antimicrob. Agents Chemother. 58(7) , 3837-42, (2014) Onychomycosis is a common fungal nail disease that is difficult to treat topically due to the deep location of the infection under the densely keratinized nail plate. Keratin affinity of topical drugs... |
|
|
Thermogelling hydrogels of cyclodextrin/poloxamer polypseudorotaxanes as aqueous-based nail lacquers: application to the delivery of triamcinolone acetonide and ciclopirox olamine.
Eur. J. Pharm. Biopharm. 83(3) , 370-7, (2013) This work investigated the use of in situ gelling hydrogels based on polypseudorotaxanes of Pluronic F-127 and partially methylated β-cyclodextrin as aqueous nail lacquers. N-acetylcysteine and urea w... |
| Ciclopirox Olamine |
| 6-cyclohexyl-1-hydroxy-4-méthylpyridin-2(1H)-one |
| Terit |
| 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone |
| MFCD00599441 |
| Ciclopirox |
| 6-Cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-one |
| Ciclopirox (USP) |
| Mycoster |
| cyclopirox |
| EINECS 249-577-2 |
| 6-Cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-on |
| Stieprox |
| Loprox |
| Ciclopiroxum |
| 1-Hydroxy-4-methyl-6-cyclohexyl-2-pyridone |
| Penlac |
| (6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone) |
| 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone |
| 2(1H)-Pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl- |
| 6-cyclohexyl-1-hydroxy-4-methyl-1,2-dihydropyridin-2-one |
| Batrafen |
| 6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one |