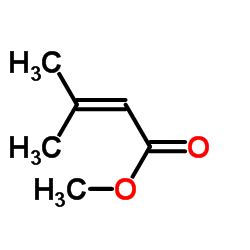
环吡酮胺
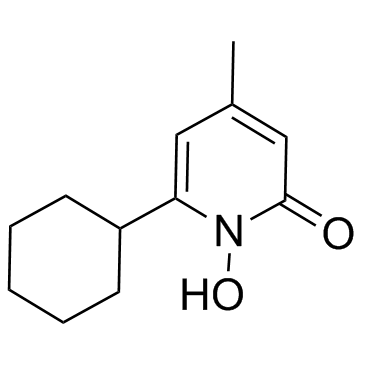
环吡酮胺结构式

|
常用名 | 环吡酮胺 | 英文名 | Ciclopirox |
|---|---|---|---|---|
| CAS号 | 29342-05-0 | 分子量 | 207.269 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 350.0±25.0 °C at 760 mmHg | |
| 分子式 | C12H17NO2 | 熔点 | 1440C | |
| MSDS | N/A | 闪点 | 165.5±23.2 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
环吡酮胺用途Ciclopirox (Penlac)是合成的抗真菌化合物。 |
| 中文名 | 环吡酮 |
|---|---|
| 英文名 | ciclopirox |
| 中文别名 | 6-环己基-1-羟基-4-甲基-2(1H)-吡啶酮 | 6-环己基-1-羟基-4-甲基-2(IH)-吡啶酮 | 环吡酮 |
| 英文别名 | 更多 |
| 描述 | Ciclopirox (Penlac)是合成的抗真菌化合物。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.2±0.1 g/cm3 |
|---|---|
| 沸点 | 350.0±25.0 °C at 760 mmHg |
| 熔点 | 1440C |
| 分子式 | C12H17NO2 |
| 分子量 | 207.269 |
| 闪点 | 165.5±23.2 °C |
| 精确质量 | 207.125931 |
| PSA | 42.23000 |
| LogP | 2.59 |
| 外观性状 | 白色或淡黄色粉末 |
| 蒸汽压 | 0.0±1.7 mmHg at 25°C |
| 折射率 | 1.582 |
| 储存条件 | -20°C Freezer |
| 分子结构 | 1、 摩尔折射率:57.96 2、 摩尔体积(cm3/mol):173.6 3、 等张比容(90.2K):465.8 4、 表面张力(dyne/cm):51.7 5、 极化率(10-24cm3):22.98 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):2 2.氢键供体数量:1 3.氢键受体数量:2 4.可旋转化学键数量:1 5.互变异构体数量:3 6.拓扑分子极性表面积40.5 7.重原子数量:15 8.表面电荷:0 9.复杂度:325 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:白色结晶性粉末,无臭,味苦。 2.熔点(ºC):144 3.溶解性:易溶于甲醇、乙醇或氯仿,略溶于二甲基甲酰胺或水,微溶于乙醚 |
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H302 |
| 警示性声明 | P301 + P312 + P330 |
| 危害码 (欧洲) | C,O |
| 风险声明 (欧洲) | R8:Contact with combustible material may cause fire. R35:Causes severe burns. R34:Causes burns. R20:Harmful by inhalation. |
| 安全声明 (欧洲) | S23-S26-S36-S45 |
| 危险品运输编码 | UN 3264 8/PG 3 |
| WGK德国 | 1 |
| RTECS号 | QU5900000 |
| 包装等级 | II |
| 危险类别 | 8 |
| 海关编码 | 2933790090 |
|
~% 
环吡酮胺 29342-05-0 |
| 文献:Hoechst Aktiengesellschaft Patent: US3972888 A1, 1976 ; |
|
~% 
环吡酮胺 29342-05-0 |
| 文献:Hoechst Aktiengesellschaft Patent: US3972888 A1, 1976 ; |
|
~% 
环吡酮胺 29342-05-0 |
| 文献:Hoechst Aktiengesellschaft Patent: US3972888 A1, 1976 ; |
|
~% 
环吡酮胺 29342-05-0 |
| 文献:Lohaus; Dittmar Arzneimittel-Forschung/Drug Research, 1981 , vol. 31, # 8 a p. 1311 - 1316 |
|
~% 
环吡酮胺 29342-05-0 |
| 文献:Lohaus; Dittmar Arzneimittel-Forschung/Drug Research, 1981 , vol. 31, # 8 a p. 1311 - 1316 |
4-甲基-3-戊烯-2-酮经次氯酸钠氧化(收率56%),再甲酯化得3-甲基-2-丁烯酸甲酯(I),环己烷羧酸和氯化亚砜反应得环己烷甲酰氯。在三氯化铝作用下,和3-甲基-2-丁烯酸甲酯(I)在二氯甲烷溶剂中,搅拌反应4h,经后处理后,真空收集140-145℃(0.4kPa)的馏分,得5-氧代-3-甲基-5-环己基-3-戊烯酸甲酯(环吡司,II),收率75%。再和盐酸羟胺、乙酸钠、甲醇及水在室温下搅拌20h,加入 50%氢氧化钠再搅拌1h。冷后用苯萃取,水相酸化至Ph=6。析出的结晶再用乙醇重结晶,即可得环吡酮.
| 海关编码 | 2933399090 |
|---|---|
| 中文概述 | 2933399090. 其他结构含非稠合吡啶环的化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途, 乌洛托品请注明外观, 6-己内酰胺请注明外观, 签约日期 |
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Effect of Wnt inhibitors in pancreatic cancer.
Anticancer Res. 34(10) , 5375-80, (2014) Activated Wnt signaling in cancer cells leads to cell proliferation. It has been shown that the Wnt pathway is activated in pancreatic adenocarcinoma cells. Therefore, we tested the effect of Wnt inhi... |
|
|
The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment.
Antimicrob. Agents Chemother. 58(7) , 3837-42, (2014) Onychomycosis is a common fungal nail disease that is difficult to treat topically due to the deep location of the infection under the densely keratinized nail plate. Keratin affinity of topical drugs... |
|
|
Thermogelling hydrogels of cyclodextrin/poloxamer polypseudorotaxanes as aqueous-based nail lacquers: application to the delivery of triamcinolone acetonide and ciclopirox olamine.
Eur. J. Pharm. Biopharm. 83(3) , 370-7, (2013) This work investigated the use of in situ gelling hydrogels based on polypseudorotaxanes of Pluronic F-127 and partially methylated β-cyclodextrin as aqueous nail lacquers. N-acetylcysteine and urea w... |
| Ciclopirox Olamine |
| 6-cyclohexyl-1-hydroxy-4-méthylpyridin-2(1H)-one |
| Terit |
| 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone |
| MFCD00599441 |
| Ciclopirox |
| 6-Cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-one |
| Ciclopirox (USP) |
| Mycoster |
| cyclopirox |
| EINECS 249-577-2 |
| 6-Cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-on |
| Stieprox |
| Loprox |
| Ciclopiroxum |
| 1-Hydroxy-4-methyl-6-cyclohexyl-2-pyridone |
| Penlac |
| (6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone) |
| 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone |
| 2(1H)-Pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl- |
| 6-cyclohexyl-1-hydroxy-4-methyl-1,2-dihydropyridin-2-one |
| Batrafen |
| 6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one |