Calcitriol
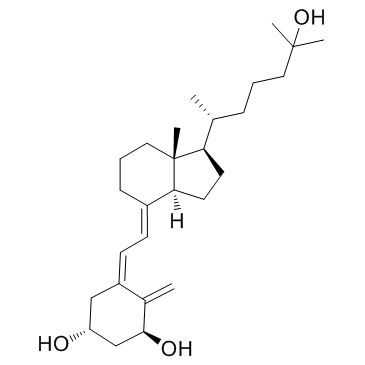
Calcitriol structure
|
Common Name | Calcitriol | ||
|---|---|---|---|---|
| CAS Number | 32222-06-3 | Molecular Weight | 416.637 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 565.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C27H44O3 | Melting Point | 119-1210C | |
| MSDS | Chinese USA | Flash Point | 238.4±24.7 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of CalcitriolCalcitriol is the most active metabolite of vitamin D and also a vitamin D receptor (VDR) agonist. |
| Name | calcitriol |
|---|---|
| Synonym | More Synonyms |
| Description | Calcitriol is the most active metabolite of vitamin D and also a vitamin D receptor (VDR) agonist. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Calcitriol exerts antiproliferative effects on cervical cancer cells in vitro. Cells decrease by 12.8% when treated with 100 nM Calcitriol for 6 days, compare with control. Inhibition of cell proliferation becomes more pronounced with the increase in Calcitriol concentration. The decrease is 26.1% and 31.6% for 200 and 500 nM Calcitriol, respectively. Treatment with Calcitriol for 72 h induces an evident accumulation of cells in the G1 phase, with approximately 66.18% in 200 nM and 78.10% in 500 nM, compare with the control (24.36%). Calcitriol treatment significantly decreases HCCR-1 protein expression compare with the control in a time- and dose-dependent manner[1]. Calcitriol significantly increases ERα mRNA in a dose dependent manner with an EC50 of 9.8×10-9 M[2]. |
| In Vivo | Chronic treatment with Calcitriol (150 ng/kg per day for 4.5 months) improves the relaxations (pD2: 6.30±0.09, Emax: 68.6±3.9% in Calcitriol-treated OVX, n=8). Renal blood flow in OVX rats is reduced in both kidneys, and the flow is restored by Calcitriol treatment. The increased expression of COX-2 and Thromboxane-prostanoid (TP) receptor in OVX rat renal arteries is reduced by chronic calcitriol administration[3]. High- and low-dose Calcitriol treatment significantly decreases the systolic blood pressure (SBP) in the fructose-fed rats by 14±4 and 9±4 mmHg, respectively, at Day 56. High-dose Calcitriol treatment (20 ng/kg per day) significantly increases serum ionized calcium level (1.44±0.05 mmol/L) compare with the other groups[4]. |
| Cell Assay | HeLa S3 cells are plated at a density of 1,000 cells/well in 96-well plates of Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), treated with 1% ethanol (control) or various concentrations of Calcitriol (100, 200, and 500 nM) for 72 h. A Cell Counting Kit8 (CCK-8) is used to determine cell proliferation. At 24, 48, 72, 96, 120, and 144 h after culturing with 200 nM Calcitriol, cells are harvested for analysis. Three independent experiments are performed in quadruplicate[1]. |
| Animal Admin | Adult female Sprague-Dawley rats weighing 200 to 220g are used in this study. Rats are housed in a temperature-controlled room (~23°C) with a 12-h light/dark cycle. The animals have free access to a standard diet and water. Ovariectomy (OVX) is performed on rats. At 6 months after the surgical procedure, the OVX rats are randomly assigned to either treatment with vehicle dimethyl sulfoxide (OVX+vehicle) or Calcitriol (150 ng/kg daily, OVX+calcitriol). Calcitriol treatment is given by oral gavage and lasted or 4.5 months. Blood pressure and serum Calcitriol level are measured[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 565.0±50.0 °C at 760 mmHg |
| Melting Point | 119-1210C |
| Molecular Formula | C27H44O3 |
| Molecular Weight | 416.637 |
| Flash Point | 238.4±24.7 °C |
| Exact Mass | 416.329041 |
| PSA | 60.69000 |
| LogP | 6.12 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |
| Index of Refraction | 1.547 |
| Stability | Air and Light Sensitive |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P280-P302 + P352 + P312-P304 + P340 + P312-P370 + P378-P403 + P235 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+:Verytoxic; |
| Risk Phrases | R26/27/28;R63 |
| Safety Phrases | S36/37/39-S45 |
| RIDADR | UN 2811 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS | FZ4645000 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| HS Code | 2942000000 |
| HS Code | 2942000000 |
|---|
|
1,25-dihydroxyvitamin D3 causes ADAM10-dependent ectodomain shedding of tumor necrosis factor receptor 1 in vascular smooth muscle cells.
Mol. Pharmacol. 87(3) , 533-42, (2015) 1,25-Dihydroxyvitamin D3 (1,25D3) has a potential antiatherosclerotic effect through anti-inflammatory actions. We investigated how 1,25D3 regulates tumor necrosis factor-α (TNF-α)-induced lectin-like... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol |
| MFCD00867079 |
| 1,3-Cyclohexanediol, 4-methylene-5-[2-[(1R,3aS,7aR)-octahydro-1-[(1R)-5-hydroxy-1,5-dimethylhexyl]-7a-methyl-4H-inden-4-ylidene]ethylidene]-, (1R,3S)- |
| (1S,3R)-9,10-Secocholesta-5,7,10-triene-1,3,25-triol |
| 1α,25-Dihydroxyvitamin D3 |
| EINECS 250-963-8 |
 CAS#:873567-12-5
CAS#:873567-12-5 CAS#:73837-24-8
CAS#:73837-24-8 CAS#:70550-73-1
CAS#:70550-73-1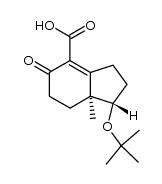 CAS#:31944-51-1
CAS#:31944-51-1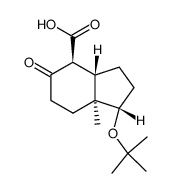 CAS#:53702-06-0
CAS#:53702-06-0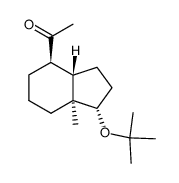 CAS#:81506-30-1
CAS#:81506-30-1
