ecdysone
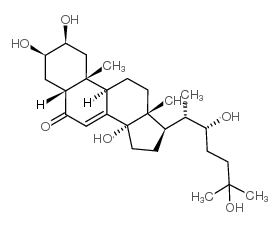
ecdysone structure
|
Common Name | ecdysone | ||
|---|---|---|---|---|
| CAS Number | 3604-87-3 | Molecular Weight | 464.63500 | |
| Density | 1.22g/cm3 | Boiling Point | 632ºC at 760mmHg | |
| Molecular Formula | C27H44O6 | Melting Point | 242°C | |
| MSDS | Chinese USA | Flash Point | 350ºC | |
Use of ecdysoneEcdysone (α-Ecdysone), a major steroid hormone in insects and herbs, triggers mineralocorticoid receptor (MR) activation and induces cellular apoptosis. Ecdysone plays essential roles in coordinating developmental transitions and homeostatic sleep regulation through its active metabolite 20-hydroxyecdysone (Crustecdysone; 20E; HY-N6979)[1][2]. |
| Name | ecdysone |
|---|---|
| Synonym | More Synonyms |
| Description | Ecdysone (α-Ecdysone), a major steroid hormone in insects and herbs, triggers mineralocorticoid receptor (MR) activation and induces cellular apoptosis. Ecdysone plays essential roles in coordinating developmental transitions and homeostatic sleep regulation through its active metabolite 20-hydroxyecdysone (Crustecdysone; 20E; HY-N6979)[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Ecdysone (α-Ecdysone; 100 nM; for 48 hours) causes renal tubular inner medullary collecting duct cells (IMCD) apoptosis[1]. Ecdysone (10, 100 nM; for 48 hours) induces the expression of a-smooth muscle actin (SMA), a standard mesenchymal marker in a dose dependent fashion in inner medullary collecting duct cells (IMCD). Ecdysone elevates the expression of cleaved caspase 3 in a dose dependent fashion[1]. Ecdysone (10, 100 nM; for 12, 24 hours) suppresses cell motility and scratch wound closure to a comparable extent[1]. Ecdysone treatments (100 nM; for 24, 48 hours) induces a branched spindle mesenchymal-like cell shape[1]. Apoptosis Analysis[1] Cell Line: Inner medullary collecting duct cells (IMCD) Concentration: 100 nM Incubation Time: For 48 hours Result: Caused renal tubular cell apoptosis. Western Blot Analysis[1] Cell Line: IMCD cells Concentration: 10, 100 nM Incubation Time: For 48 hours Result: Induced the expression of a-smooth muscle actin (SMA), a standard mesenchymal marker in a dose dependent fashion. |
| In Vivo | Ecdysone (α-Ecdysone; 6 µg/g/day; SC; for 14 days) evidently impaires kidney function marked by a statistically significant increase in BUN levels and amplifies renal expression of α-SMA in male C57BL/6 mice aged 10 weeks. Ecdysone confers an MR dependent nephropathic effect[1]. |
| References |
| Density | 1.22g/cm3 |
|---|---|
| Boiling Point | 632ºC at 760mmHg |
| Melting Point | 242°C |
| Molecular Formula | C27H44O6 |
| Molecular Weight | 464.63500 |
| Flash Point | 350ºC |
| Exact Mass | 464.31400 |
| PSA | 118.22000 |
| LogP | 2.73910 |
| Index of Refraction | 1.582 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | FZ8170000 |
| HS Code | 2937290090 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Chemical & Pharmaceutical Bulletin, , vol. 29, # 5 p. 1445 - 1451 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
|
~% 
ecdysone CAS#:3604-87-3 |
| Literature: Tetrahedron Letters, , vol. 21, # 45 p. 4323 - 4326 |
| HS Code | 2937290090 |
|---|
|
A crayfish molar tooth protein with putative mineralized exoskeletal chitinous matrix properties.
J. Exp. Biol. 218 , 3487-98, (2015) Some crustaceans possess exoskeletons that are reinforced with calcium carbonate. In the crayfish Cherax quadricarinatus, the molar tooth, which is part of the mandibular exoskeleton, contains an unus... |
|
|
The silkworm glutathione S-transferase gene noppera-bo is required for ecdysteroid biosynthesis and larval development.
Insect Biochem. Mol. Biol. 61 , 1-7, (2015) Insect molting and metamorphosis are tightly controlled by ecdysteroids, which are important steroid hormones that are synthesized from dietary sterols in the prothoracic gland. One of the ecdysteroid... |
|
|
Genome-wide promoter binding profiling of protein phosphatase-1 and its major nuclear targeting subunits.
Nucleic Acids Res. 43 , 5771-84, (2015) Protein phosphatase-1 (PP1) is a key regulator of transcription and is targeted to promoter regions via associated proteins. However, the chromatin binding sites of PP1 have never been studied in a sy... |
| MFCD00042683 |
| A-ECDYSONE |
| [3H]-Ecdysone |
| Ecdysone |
| EINECS 222-760-4 |
| (2β,3β,5β,22R)-2,3,14,22,25-pentahydroxycholest-7-en-6-one |
| Gynostemma Gypenosides |
| α-Ecdysone |
![(2aS,4R,5S,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bR)-10-(3-methoxy-3-oxopropyl)-6a,8a,9-trimethyl-2-oxooctadecahydro-1H-naphtho[2',1':4,5]indeno[2,1-b]furan-4,5-diyl diacetate structure](https://image.chemsrc.com/caspic/076/77260-31-2.png)
![(2aS,4R,5S,6aR,6bR,8aS,8bR,9S,10R,11aS,12aR)-10-(3-methoxy-3-oxopropyl)-6a,8a,9-trimethyl-2-oxo-2a,3,4,5,6,6a,6b,7,8,8a,8b,9,10,11a,12,12a-hexadecahydro-2H-naphtho[2',1':4,5]indeno[2,1-b]furan-4,5-diyl diacetate structure](https://image.chemsrc.com/caspic/000/77260-33-4.png)
![(2aS,4R,5S,6aR,6bR,8aR,8bR,9S,10R,11aS)-10-(3-methoxy-3-oxopropyl)-6a,8a,9-trimethyl-2-oxo-2a,3,4,5,6,6a,6b,7,8,8a,8b,9,10,11a-tetradecahydro-2H-naphtho[2',1':4,5]indeno[2,1-b]furan-4,5-diyl diacetate structure](https://image.chemsrc.com/caspic/462/77260-34-5.png)
![(1S,2aS,4R,5S,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bR)-1-bromo-10-(3-methoxy-3-oxopropyl)-6a,8a,9-trimethyl-2-oxooctadecahydro-1H-naphtho[2',1':4,5]indeno[2,1-b]furan-4,5-diyl diacetate structure](https://image.chemsrc.com/caspic/038/77260-32-3.png)
![(2aS,4R,5S,6aR,6bR,8aR,8bR,9S,10R,11aS,12aS)-12a-hydroxy-10-(3-methoxy-3-oxopropyl)-6a,8a,9-trimethyl-2-oxo-2a,3,4,5,6,6a,6b,7,8,8a,8b,9,10,11a,12,12a-hexadecahydro-2H-naphtho[2',1':4,5]indeno[2,1-b]furan-4,5-diyl diacetate structure](https://image.chemsrc.com/caspic/054/77273-84-8.png)

![(2S,3R,5S,9R,10R,13R,17R)-10,13-dimethyl-6-oxo-17-((S)-1-((R)-5-oxotetrahydrofuran-2-yl)ethyl)-2,3,4,5,6,7,9,10,11,12,13,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-2,3-diyl diacetate structure](https://image.chemsrc.com/caspic/386/77260-36-7.png)
![(2S,3R,5S,9R,10R,13R,14S,17R)-14-hydroperoxy-10,13-dimethyl-6-oxo-17-((S)-1-((R)-5-oxotetrahydrofuran-2-yl)ethyl)-2,3,4,5,6,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-2,3-diyl diacetate structure](https://image.chemsrc.com/caspic/348/77260-37-8.png)
![(2S,3R,5S,10R,13R,14S,17R)-14-hydroxy-10,13-dimethyl-6-oxo-17-((S)-1-((R)-5-oxotetrahydrofuran-2-yl)ethyl)-2,3,4,5,6,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-2,3-diyl diacetate structure](https://image.chemsrc.com/caspic/023/19327-83-4.png)

