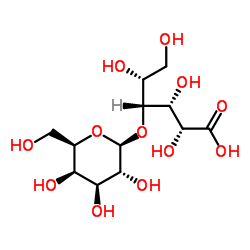
n-2,10-dion(1:1)
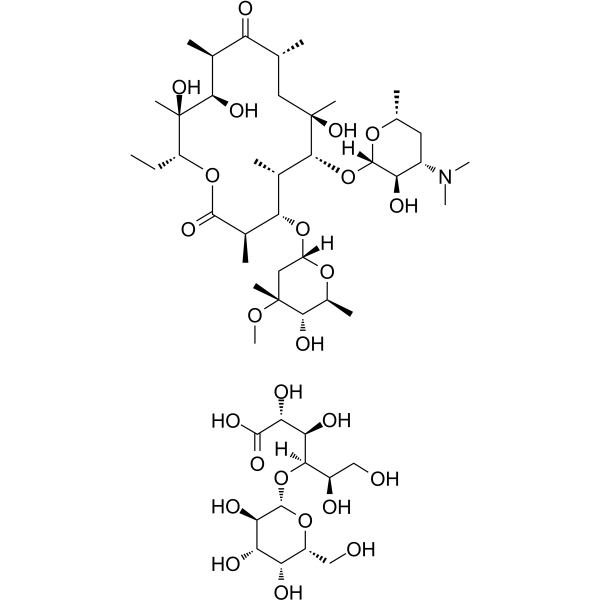
n-2,10-dion(1:1) structure
|
Common Name | n-2,10-dion(1:1) | ||
|---|---|---|---|---|
| CAS Number | 3847-29-8 | Molecular Weight | 1092.223 | |
| Density | 0.9083 (rough estimate) | Boiling Point | 818.4ºC at 760mmHg | |
| Molecular Formula | C49H89NO25 | Melting Point | 145-150° | |
| MSDS | N/A | Flash Point | 448.8ºC | |
Use of n-2,10-dion(1:1)Erythromycin lactobionate is a macrolide antibiotic produced by actinomycete Streptomyces erythreus with a broad spectrum of antimicrobial activity. Erythromycin lactobionate binds to bacterial 50S ribosomal subunits and inhibits RNA-dependent protein synthesis by blockage of transpeptidation and/or translocation reactions, without affecting synthesis of nucleic acid[1][2]. Erythromycin lactobionate also exhibits antitumor and neuroprotective effect in different fields of research[3][4]. |
| Name | erythromycin lactobionate (200 mg) |
|---|---|
| Synonym | More Synonyms |
| Description | Erythromycin lactobionate is a macrolide antibiotic produced by actinomycete Streptomyces erythreus with a broad spectrum of antimicrobial activity. Erythromycin lactobionate binds to bacterial 50S ribosomal subunits and inhibits RNA-dependent protein synthesis by blockage of transpeptidation and/or translocation reactions, without affecting synthesis of nucleic acid[1][2]. Erythromycin lactobionate also exhibits antitumor and neuroprotective effect in different fields of research[3][4]. |
|---|---|
| Related Catalog | |
| Target |
Bacterial; RNA-dependent protein synthesis[1] |
| In Vitro | Erythromycin lactobionate inhibits growth of P. falciparum with IC50 and IC90 values of 58.2 μM and 104.0 μM, respectively[1]. Erythromycin lactobionate (10 μM, 100 μM; 24 h, 72 h) shows antioxidant and anti-inflammatory effects and suppresses the accumulation of 4-HNE (p<0.01) and 8-OHdG (p<0.01), reduces Iba-1 (p<0.01) and TNF-α (p<0.01) expression significantly[4]. Cell Viability Assay[4] Cell Line: Embryos primary cortical neuron (from the cerebral cortices of 17-day-old Sprague-Dawley rat) Concentration: 10, 100 μM Incubation Time: 24, 72 hours Result: Improved the viability of cultured neuronal cells in vitro after 3 hours oxygen-glucose deprivation (OGD). |
| In Vivo | Erythromycin lactobionate (gastric intubation; 0.1-50 mg/kg; 30-120 days) decreases tumor growth and prolong the survival time of mice from dose of 5 mg/kg in mice[3]. Erythromycin lactobionate (gastric intubation; 5 mg/kg) protects mice alive even at 120 days after inoculation, but shortens mean survival time in tumor-bearing mice by 4-5 days with dose of 50 mg/kg[3]. Erythromycin lactobionate (i.h.; single injection; 50 mg/kg) has a protective effect on the rat model with cerebral ischemia reperfusion-injury[4]. Animal Model: Female ddY mice (6 week-old) with EAC cells or CDF mice (6 week-old) with P388 cells[3] Dosage: 0.1 mg/kg; 0.5 mg/kg; 10 mg/kg; 30 mg/kg; 50 mg/kg Administration: Gastric intubation; 30-120 days Result: Decreased tumor growth and prolonged the mean survival time of mice from the dose of 5 mg/kg, however, the 50 mg/kg dosage shortened the MST in tumorbearing mice. Animal Model: Male Sprague-Dawley rats (8-week-old, 250-300 g)[4] Dosage: 50 mg/kg Administration: Subcutaneous single injection Result: Reduced infarct volume and edema volume, improved neurological deficit. |
| References |
[1]. Gribble MJ, et al. Erythromycin. Med Clin North Am. 1982 Jan;66(1):79-89. [3]. Hamada K, et al. Antitumor effect of erythromycin in mice. Chemotherapy. 1995 Jan-Feb. 41(1):59-69. |
| Density | 0.9083 (rough estimate) |
|---|---|
| Boiling Point | 818.4ºC at 760mmHg |
| Melting Point | 145-150° |
| Molecular Formula | C49H89NO25 |
| Molecular Weight | 1092.223 |
| Flash Point | 448.8ºC |
| Exact Mass | 1091.572388 |
| PSA | 411.51000 |
| Index of Refraction | 1.5300 (estimate) |
| Storage condition | 20°C |
| Water Solubility | Soluble in water, freely soluble in anhydrous ethanol and in methanol, very slightly soluble in acetone and in methylene chloride. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| RIDADR | NONH for all modes of transport |
|---|
|
~98% 
n-2,10-dion(1:1) CAS#:3847-29-8 |
| Literature: Liu, Li Patent: EP2301945 A1, 2011 ; Location in patent: Page/Page column 9-10 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| decane-2,10-dione (1:1) |
| Erythrocin lactobionate |
| (2R,3R,4R,5R)-2,3,5,6-Tetrahydroxy-4-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}hexanoic acid - (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione (1:1) (non-preferred name) |
| ERYTHROMYCIN LACTOBIONATE USP(CRM STANDARD) |
| lactobionic acid,compd. with erythromycin |
| Azithromycin lactobionate |
| (2R,3R,4R,5R)-2,3,5,6-Tetrahydroxy-4-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}hexanoic acid - (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione (1:1) |
| Erythromycin,lactobionate (1:1) (salt) |
| 4-O-b-D-Galactopyranosyl-D-gluconic Acid compd. with Erythromycin (1:1) |
| Erythomycin lactobionate |
| lactobionicacid,compd.witherythromycin(1:1) |
| tradécane-2,10-dione (1:1) |
| decane-2,10-dione (1:1) (non-preferred name) |
| Erythromycin,compd. with lactobionic acid (7CI) |
| acide (2R,3R,4R,5R)-2,3,5,6-tétrahydroxy-4-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}hexanoïque - (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2S,3R,4S,6R)-4-(diméthylamino)-3-hydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-14-éthyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-méthoxy-4,6-diméthyltétrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13-hexaméthyloxacyclotétradécane-2,10-dione (1:1) |
| n-2,10-dion(1:1) |
| erythromycin lactobionate |
| (2R,3R,4R,5R)-2,3,5,6-Tetrahydroxy-4-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}hexansäure--(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2,10-dion(1:1) |