Aurintricarboxylic acid
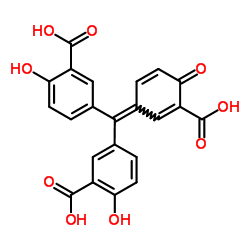
Aurintricarboxylic acid structure
|
Common Name | Aurintricarboxylic acid | ||
|---|---|---|---|---|
| CAS Number | 4431-00-9 | Molecular Weight | 422.341 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 759.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C22H14O9 | Melting Point | 300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 427.1±29.4 °C | |
Use of Aurintricarboxylic acidAurintricarboxylic acid is a nanomolar-potency, allosteric antagonist with selectivity towards αβ-methylene-ATP-sensitive P2X1Rs and P2X3Rs, with IC50s of 8.6 nM and 72.9 nM for rP2X1R and rP2X3R, respectively[1]. Aurintricarboxylic acid is a potent anti-influenza agent by directly inhibiting the neuraminidase[2]. Aurintricarboxylic acid is an inhibitor of topoisomerase II and apoptosis[3]. Aurintricarboxylic acid is a selective inhibitor of the TWEAK-Fn14 signaling pathway[4]. |
| Name | aurintricarboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Aurintricarboxylic acid is a nanomolar-potency, allosteric antagonist with selectivity towards αβ-methylene-ATP-sensitive P2X1Rs and P2X3Rs, with IC50s of 8.6 nM and 72.9 nM for rP2X1R and rP2X3R, respectively[1]. Aurintricarboxylic acid is a potent anti-influenza agent by directly inhibiting the neuraminidase[2]. Aurintricarboxylic acid is an inhibitor of topoisomerase II and apoptosis[3]. Aurintricarboxylic acid is a selective inhibitor of the TWEAK-Fn14 signaling pathway[4]. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase II rP2X1R:8.6 nM (IC50) rP2X3R:72.9 nM (IC50) Apoptosis |
| In Vitro | Aurintricarboxylic acid strongly inhibits rP2X1Rs and rP2X3Rs with IC50 values of 8.6 nM and 72.9 nM, respectively, and more weakly inhibits P2X2/3Rs, P2X2Rs, P2X4Rs or P2X7Rs[1]. Aurintricarboxylic acid inhibition of ATP-induced currents is concentration dependent[1]. Aurintricarboxylic acid is a non-competitive antagonist of P2X3R[1]. Aurintricarboxylic acid can inhibit the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) and vaccinia virus[2]. Aurintricarboxylic acid protects MDCK cells from infection with influenza A strains PR8, NY or N[2]. Aurintricarboxylic acid inhibits replication of influenza A and B viruses[2]. Aurintricarboxylic acid inhibits neuraminidase activities of influenza A and B viruses[2]. Aurintricarboxylic acid inhibits TWEAK-Fn14-mediated NF-κB activation[4]. Aurintricarboxylic acid (10 μM;0.5-2 hours) suppresses TWEAK-Fn14-mediated NF-κB, Akt, and Src phosphorylation in GBM cells[4]. Aurintricarboxylic acid blocks TWEAK-stimulated Rac1 activation and TRAF2 recruitment to Fn14 cytoplasmic domain in glioma cells[4]. Aurintricarboxylic acid represses TWEAK-stimulated glioma cell migration and invasion without causing cell cytotoxicity[4]. Western Blot Analysis[4] Cell Line: T98G, A172, GBM44 glioma cells Concentration: 10 μM Incubation Time: 0.5 hour, 1 hour, 2 hours Result: Abrogated TWEAK activation of downstream signals including phosphorylation of the NF-κB family member p65, Akt, and Src in all three GBM cell lines. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 759.6±60.0 °C at 760 mmHg |
| Melting Point | 300 °C(lit.) |
| Molecular Formula | C22H14O9 |
| Molecular Weight | 422.341 |
| Flash Point | 427.1±29.4 °C |
| Exact Mass | 422.063782 |
| PSA | 169.43000 |
| LogP | 4.09 |
| Appearance of Characters | powder | dark red to brown |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.751 |
| Water Solubility | 1 M NH4OH: 10 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | GU4790000 |
|
Altered Oligodendrocyte Maturation and Myelin Maintenance: The Role of Antiretrovirals in HIV-Associated Neurocognitive Disorders.
J. Neuropathol. Exp. Neurol. 74 , 1093-118, (2015) Despite effective viral suppression through combined antiretroviral therapy (cART), approximately half of HIV-positive individuals have HIV-associated neurocognitive disorders (HAND). Studies of antir... |
|
|
MicroRNA-23a has minimal effect on endurance exercise-induced adaptation of mouse skeletal muscle.
Pflugers Arch. 467(2) , 389-98, (2015) Skeletal muscles contain several subtypes of myofibers that differ in contractile and metabolic properties. Transcriptional control of fiber-type specification and adaptation has been intensively inve... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
| Benzoic acid, 5-((3-carboxy-4-hydroxyphenyl)(3-carboxy-4-oxo-2,5-cyclohexadien-1-ylidene)methyl)-2-hydroxy- |
| 3,3'-[(3-Carboxy-4-oxocyclohexa-2,5-dien-1-ylidene)methylene]bis(6-hydroxybenzoic acid) |
| Aurintricarboxylic acid |
| 3,3'-[(3-carboxy-4-oxocyclohexa-2,5-dien-1-ylidene)methanediyl]bis(6-hydroxybenzoic acid) |
| EINECS 224-628-1 |
| 3,3'-[(3-Carboxy-4-oxo-2,5-cyclohexadien-1-ylidene)methylene]bis(6-hydroxybenzoic acid) |
| Benzoic acid, 3,3'-[(3-carboxy-4-oxo-2,5-cyclohexadien-1-ylidene)methylene]bis[6-hydroxy- |
| MFCD00011663 |