Proscillaridin A
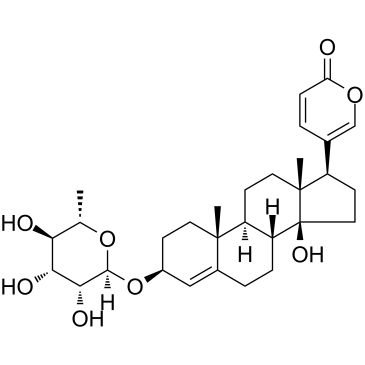
Proscillaridin A structure
|
Common Name | Proscillaridin A | ||
|---|---|---|---|---|
| CAS Number | 466-06-8 | Molecular Weight | 530.650 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 716.7±60.0 °C at 760 mmHg | |
| Molecular Formula | C30H42O8 | Melting Point | 233ºC | |
| MSDS | Chinese USA | Flash Point | 232.6±26.4 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Proscillaridin AProscillaridin A is a potent poison of topoisomerase I/II activity with IC50 values of 30 nM and 100 nM, respectively[1]. |
| Name | Proscillaridin A |
|---|---|
| Synonym | More Synonyms |
| Description | Proscillaridin A is a potent poison of topoisomerase I/II activity with IC50 values of 30 nM and 100 nM, respectively[1]. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I:30 nM (IC50) Topoisomerase II:100 nM (IC50) |
| In Vitro | Proscillaridin A (0-160 nM; 48 hours) reveals the antiproliferative response in a time- as well as dose-dependent manner in MCF-7 cells[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 716.7±60.0 °C at 760 mmHg |
| Melting Point | 233ºC |
| Molecular Formula | C30H42O8 |
| Molecular Weight | 530.650 |
| Flash Point | 232.6±26.4 °C |
| Exact Mass | 530.287964 |
| PSA | 129.59000 |
| LogP | 3.90 |
| Vapour Pressure | 0.0±5.2 mmHg at 25°C |
| Index of Refraction | 1.620 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | 28-45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | UK6650000 |
|
Pulse pressure correlates in humans with a proscillaridin A immunoreactive compound.
Hypertension 27(5) , 1073-8, (1996) Endogenous digitalis-like factors in humans are presumably cardenolides and bufadienolides. To test whether bufadienolide-like substances may circulate in human blood, we used antibodies from rabbits ... |
|
|
Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs.
J. Nat. Prod. 72 , 1969-74, (2009) Cardiac glycosides have been reported to exhibit cytotoxic activity against several different cancer types, but studies against colorectal cancer are lacking. In a screening procedure aimed at identif... |
|
|
Role of endogenous cardiac glycosides in the spontaneously hypertensive rat--antagonism by active immunization.
Am. J. Hypertens. 9(1) , 81-5, (1996) The effects of simultaneous active immunization against two cardiac glycoside drugs, digoxin and proscillaridin, have been examined in young spontaneously hypertensive and Wistar-Kyoto rats. Control a... |
| Bufa-4,20,22-trienolide, 3-[(6-deoxy-α-L-mannopyranosyl)oxy]-14-hydroxy-, (3β)- |
| Protasin |
| 14-Hydroxy-3b-(rhamnosyloxy)bufa-4,20,22-trienolide |
| (3β)-3-[(6-Deoxy-α-L-mannopyranosyl)oxy]-14-hydroxybufa-4,20,22-trienolid |
| Scillarenin 3β-rhamnoside |
| 3-β-((6-Deoxy-α-L-mannopyranosyl)oxy)-14-hydroxybufa-4,20,22-trienolide |
| 5-[(3S,8R,9S,10R,13R,14S,17R)-14-Hydroxy-10,13-dimethyl-3-{[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2H-pyran-2-on |
| Desgluco-transvaalin |
| Urgilan |
| Herzo proscillan |
| Scillarenin 3b-Rhamnoside |
| Scillarenin 3a-L-Rhamnopyranoside |
| 3b-Rhamnosido-14b-hydroxy-D4,20,22-bufatrienolide |
| Proscillaridin |
| Scillacrist |
| Desglucotransvaaline |
| Coratol |
| Scilla "didier" |
| (3β)-3-[(6-Deoxy-α-L-mannopyranosyl)oxy]-14-hydroxybufa-4,20,22-trienolide |
| Purosin-TC |
| Sandoscill |
| Wirnesin |
| Caratol |
| (3b)-3-[(6-Deoxy-a-L-mannopyranosyl)oxy]-14-hydroxybufa-4,20,22-trienolide |
| 3b-[(6-Deoxy-a-L-mannopyranosyl)oxy]-14-hydroxybufa-4,20,22-trienolide |
| Tradenal |
| Herzo |
| Bufa-4,20,22-trienolide, 3-((6-deoxy-α-L-mannopyranosyl)oxy)-14-hydroxy-, (3β)- |
| psc-801 |
| Caradrin |
| Cardion |
| 14-Hydroxy-3β-(rhamnosyloxy)bufa-4,20,22-trienolide |
| Cardiovite |
| Prostosin |
| Proslladin |
| Solestril |
| Stellarid |
| proszin |
| proscillaridin-A |
| Talucard |
| Carmazon |
| Simeon |
| Procilan |
| 5-[(3S,8R,9S,10R,13R,14S,17R)-14-Hydroxy-10,13-dimethyl-3-{[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2H-pyran-2-one |
| Talusin |
| 5-[(3S,8R,9S,10R,13R,14S,17R)-14-Hydroxy-10,13-diméthyl-3-{[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tétradécahydro-1H-cyclopenta[a]phénanthrén-17-yl]-2H-pyran-2-one |
| Procardin |
| Caradrine |
| Proscillan |

