4-Hydroxyphenyl ethanol
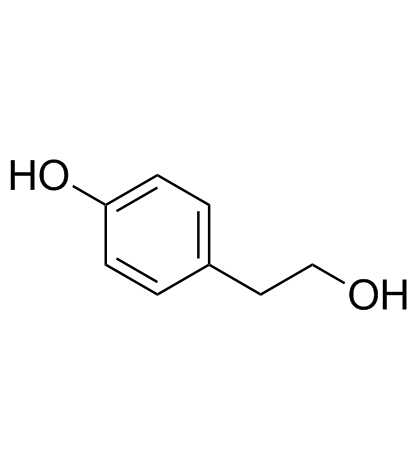
4-Hydroxyphenyl ethanol structure
|
Common Name | 4-Hydroxyphenyl ethanol | ||
|---|---|---|---|---|
| CAS Number | 501-94-0 | Molecular Weight | 138.16 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 375.2±27.0 °C at 760 mmHg | |
| Molecular Formula | C8H10O2 | Melting Point | 89-92 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 180.7±23.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-Hydroxyphenyl ethanolTyrosol is a derivative of phenethyl alcohol. Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation. Anti-oxidative and anti-inflammatory effects[1]. |
| Name | 2-(4-hydroxyphenyl)ethanol |
|---|---|
| Synonym | More Synonyms |
| Description | Tyrosol is a derivative of phenethyl alcohol. Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation. Anti-oxidative and anti-inflammatory effects[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Tyrosol (1.6 mM) significantly increases the cell viability of cultured astrocytes exposed to oxygen glucose deprivation (OGD)[1]. Tyrosol (1.6 mM) attenuates the released TNF-α and IL-6 level from astrocyte via regulating Janus N-terminal kinase (JNK) [1]. The reduction of cytokines from astrocyte might be due to its inhibition of astrocyte activation and regulation of STAT3 signaling pathway since Tyrosol (1.6 mM) attenuates the expression level of GFAP (glial fibrillary acidic protein) and the phosphorylation of STAT3[1]. Tyrosol prevents the degradation of IκBα and the increase of IκBα phosphorylation in astrocytes exposed to OGD, which leads to the suppression of NF-κB function during ischemia[1]. |
| In Vivo | Sub-plantar injection of carrageenan causes a noticeable increase in paw thickness, reaching a peak after 2 h post-injection. This effect is reduced when Tyrosol (0.5 mg/kg) or Tyrosol-sulphate is injected prior to the treatment with carrageenan. Similar AUC values for paw oedema are obtained after the administration of Tyrosol at a dose of 0.5 mg/kg and Tyrosol-sulphate at a dose of 0.1 mg/kg. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 375.2±27.0 °C at 760 mmHg |
| Melting Point | 89-92 °C(lit.) |
| Molecular Formula | C8H10O2 |
| Molecular Weight | 138.16 |
| Flash Point | 180.7±23.7 °C |
| PSA | 40.46000 |
| LogP | 0.04 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.598 |
| Water Solubility | slightly soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2907299090 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
| HS Code | 2907299090 |
|---|---|
| Summary | 2907299090 polyphenols; phenol-alcohols。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:5.5%。general tariff:30.0% |
|
Effect of different aging techniques on the polysaccharide and phenolic composition and sensory characteristics of Syrah red wines fermented using different yeast strains.
Food Chem. 179 , 116-26, (2015) The effect of high levels of the polysaccharide Saccharomyces cerevisiae yeast strain (HPS) and another conventional yeast strain (FERM) on the polysaccharide and phenolic composition of Syrah red win... |
|
|
Effect of selected Saccharomyces cerevisiae yeast strains and different aging techniques on the polysaccharide and polyphenolic composition and sensorial characteristics of Cabernet Sauvignon red wines.
J. Sci. Food Agric. 95 , 2132-44, (2015) The objective of this work was to study the effect of two Saccharomyces cerevisiae yeast strains with different capabilities of polysaccharide liberation during alcoholic fermentation in addition to s... |
|
|
Multicommuted flow injection method for fast photometric determination of phenolic compounds in commercial virgin olive oil samples.
Talanta 147 , 531-6, (2015) A multicommuted flow injection method has been developed for the determination of phenolic species in virgin olive oil samples. The method is based on the inhibitory effect of antioxidants on a stable... |
| 4-(2-Hydroxyethyl)phenol |
| 2-(4-Hydroxyphenyl)ethyl Alcohol |
| 4-Hydroxyphenylethanol |
| β-(4-Hydroxyphenyl)ethanol |
| 4-[(2S)-2-Amino-3-hydroxypropyl]phenol |
| (S)-4-(2-Amino-3-hydroxypropyl)phenol |
| b-(4-Hydroxyphenyl)ethanol |
| Benzenepropanol, β-amino-4-hydroxy-, (βS)- |
| tyrosinol |
| 4-(2-Hydroxyethyl)benzolol |
| phenethyl alcohol, 4-hydroxy- |
| p-Hydroxyphenethyl alcohol |
| Benzenepropanol, β-amino-4-hydroxy-, (S)- |
| UNII-1AK4μ3SNX |
| 2-(4-Hydroxyphenyl)ethanol |
| Ethanol, 2-(4-hydroxyphenyl)- |
| 2-(4-Hydroxyphenyl)ethan-1-ol |
| (S)-b-Amino-4-hydroxybenzenepropanol |
| 4-hydroxybenzeneethanol |
| MFCD00002902 |
| β-(p-Hydroxyphenyl)ethanol |
| 4-Hydroxyphenethyl alcohol |
| EINECS 207-930-8 |
| Benzeneethanol, 4-hydroxy- |
| p-Hydroxyphenylethanol |
| 4-Hydroxyphenethyl alcohol,Tyrosol,p-HPEA |
| 4-Hydroxyphenylethyl alcohol |
| b-(p-Hydroxyphenyl)ethanol |
| 4-Hydroxyphenyl ethanol |
| Tyrosol |
 CAS#:156-38-7
CAS#:156-38-7 CAS#:14199-15-6
CAS#:14199-15-6 CAS#:60-12-8
CAS#:60-12-8![4-[2-(2-chloroacetyl)oxoethyl]phenyl-2-chloroacetate Structure](https://image.chemsrc.com/caspic/042/1223454-88-3.png) CAS#:1223454-88-3
CAS#:1223454-88-3 CAS#:17138-28-2
CAS#:17138-28-2 CAS#:104-01-8
CAS#:104-01-8 CAS#:14140-15-9
CAS#:14140-15-9 CAS#:60-18-4
CAS#:60-18-4 CAS#:35897-92-8
CAS#:35897-92-8 CAS#:110005-81-7
CAS#:110005-81-7 CAS#:40167-10-0
CAS#:40167-10-0 CAS#:56718-71-9
CAS#:56718-71-9 CAS#:181115-01-5
CAS#:181115-01-5 CAS#:28145-35-9
CAS#:28145-35-9 CAS#:93221-48-8
CAS#:93221-48-8 CAS#:81024-42-2
CAS#:81024-42-2 CAS#:836-43-1
CAS#:836-43-1
