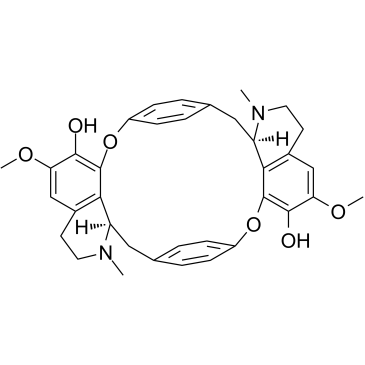
Cycleanine
Modify Date: 2024-01-02 18:57:08

Cycleanine structure
|
Common Name | Cycleanine | ||
|---|---|---|---|---|
| CAS Number | 518-94-5 | Molecular Weight | 622.75 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 691.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C38H42N2O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 169.8±28.7 °C | |
Use of CycleanineCycleanine is a potent vascular selective Calcium antagonist. Cycleanine has analgesic, muscle relaxant and anti-inflammatory activities. Cycleanine has potential for anti-ovarian cancer acting through the apoptosis pathway[1][2]. |
| Name | Cycleanine |
|---|---|
| Synonym | More Synonyms |
| Description | Cycleanine is a potent vascular selective Calcium antagonist. Cycleanine has analgesic, muscle relaxant and anti-inflammatory activities. Cycleanine has potential for anti-ovarian cancer acting through the apoptosis pathway[1][2]. |
|---|---|
| Related Catalog | |
| Target |
L-type calcium channel |
| In Vitro | Cycleanine inhibits L-type Ca-current (ICaL) of single rat ventricular cardiomyocytes in a voltage- and frequency-dependent manner[1]. Cycleanine shows modestly less potency against human OSE cells (normal) than the cancer cells[2]. Cycleanine (20 μM; 48 hours) exhibits cytotoxicity for Ovcar-8, A2780, Igrov-1, and Ovcar-4 cell lines with IC50s ranging from 7 to 14 μM[2]. Cycleanine (20 μM; 24 hours) results in significant PARP cleavage (a marker of apoptosis)[2]. Cycleanine (20 μM; 48 hours) causes a significant increase of the population of both early and late apoptotic cells[2]. Cell Cytotoxicity Assay[2] Cell Line: Ovcar-8 cells, A2780 cells, Igrov-1 cells, Ovcar-4 cells Concentration: 20 μM Incubation Time: 48 hours Result: Exhibited cytotoxicity for Ovcar-8, A2780, Igrov-1, and Ovcar-4 cell lines with IC50 values of 10 μM, 7.6 μM, 14 μM, 7.2 μM, respectively. Western Blot Analysis[2] Cell Line: Ovcar-8 cells Concentration: 20 μM Incubation Time: 24 hours Result: Induced 1.1-fold increase in PARP-1 cleavage compared with carboplatin. Apoptosis Analysis[2] Cell Line: Ovcar-8 cells Concentration: 20 μM Incubation Time: 48 hours Result: Caused a significant increase of the population of both early and late apoptotic cells. Cell Cycle Analysis[2] Cell Line: Ovcar-8 cells Concentration: 20 μM Incubation Time: 48 hours Result: Increased the percentage of Ovcar-8 cells in subG1. |
| In Vivo | Cycleanine inhibits the KCl-induced contraction of rabbit aortic rings with an IC50 of 0.8 nM[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 691.6±55.0 °C at 760 mmHg |
| Molecular Formula | C38H42N2O6 |
| Molecular Weight | 622.75 |
| Flash Point | 169.8±28.7 °C |
| Exact Mass | 622.304260 |
| PSA | 61.86000 |
| LogP | 3.96 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.586 |
|
~% 
Cycleanine CAS#:518-94-5 |
| Literature: Bhakuni,Dewan S.; Jain,Sudha; Chaturvedi,Rekha Tetrahedron, 1987 , vol. 43, # 17 p. 3975 - 3982 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| 2-Furanol,tetrahydro-3,4-dipiperonyl |
| tetrahydro-3,4-dipiperonylfuran-2-ol |
| Cubebine |
| (12ar,24ar)-5,6,17,18-tetramethoxy-1,13-dimethyl-2,3,12a,13,14,15,24,24a-octahydro-1h,12h-8,11:20,23-dietheno[1,10]dioxacyclooctadecino[13,12,11-ij:4,3,2-i'j']diisoquinoline |
| 8,11:20,23-Dietheno-1H,12H-[1,10]dioxacyclooctadecino[13,12,11-ij:4,3,2-i'j']diisoquinoline, 2,3,12a,13,14,15,24,24a-octahydro-5,6,17,18-tetramethoxy-1,13-dimethyl-, (12aR,24aR)- |
| 7,11-di-O-methylisochondodendrine |
| Cycleanine |
| 6,7,6',7'-tetramethoxy-2,2'-dimethyl-cycleanane |
| (11R,26R)-4,5,19,20-Tetramethoxy-10,25-dimethyl-2,17-dioxa-10,25-diazaheptacyclo[26.2.2.2.1.1.0.0]hexatriaconta-1(30),3(36),4,6,13,15,18(33),19,21,28,31,34-dodecaene |
| O,O'-dimethyl-isochondrodendrine |

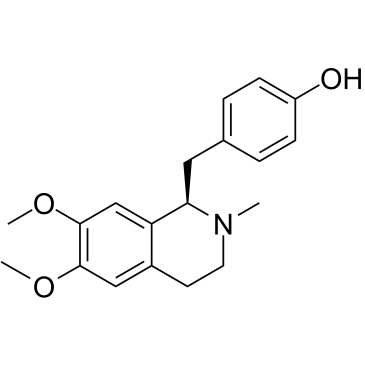 CAS#:524-20-9
CAS#:524-20-9
