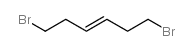
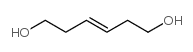
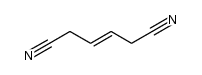
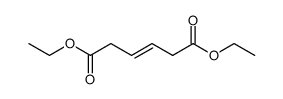
Hexamethonium bromide
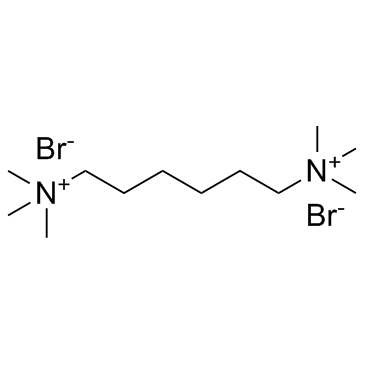
Hexamethonium bromide structure
|
Common Name | Hexamethonium bromide | ||
|---|---|---|---|---|
| CAS Number | 55-97-0 | Molecular Weight | 362.188 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C12H30Br2N2 | Melting Point | ~285 °C (dec.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Hexamethonium bromideHexamethonium is a non-depolarising ganglionic blocker, a nicotinic nACh (NN) receptor antagonist.Target: nAChRHexamethonium is a non-depolarising ganglionic blocker, a nicotinic nACh receptor antagonist that acts in autonomic ganglia by binding mostly in or on the NN receptor, and not the acetylcholine binding site itself. It does not have any effect on the muscarinic acetylcholine receptors (mAChR) located on target organs of the parasympathetic nervous system but acts as antagonist at the nicotinic acetylcholine receptors located in sympathetic and parasympathetic ganglia (NN). Hexamethonium bromide is a nicotinic acetyl choline receptor antagonist. Induces apoptosis and inhibits the stimulatory effect of nicotine on endothelial cell DNA synthesis and proliferation. Hexamethonium bromide hydrate is an inhibitor of AChR α3 [1-3]. |
| Name | hexamethonium bromide |
|---|---|
| Synonym | More Synonyms |
| Description | Hexamethonium is a non-depolarising ganglionic blocker, a nicotinic nACh (NN) receptor antagonist.Target: nAChRHexamethonium is a non-depolarising ganglionic blocker, a nicotinic nACh receptor antagonist that acts in autonomic ganglia by binding mostly in or on the NN receptor, and not the acetylcholine binding site itself. It does not have any effect on the muscarinic acetylcholine receptors (mAChR) located on target organs of the parasympathetic nervous system but acts as antagonist at the nicotinic acetylcholine receptors located in sympathetic and parasympathetic ganglia (NN). Hexamethonium bromide is a nicotinic acetyl choline receptor antagonist. Induces apoptosis and inhibits the stimulatory effect of nicotine on endothelial cell DNA synthesis and proliferation. Hexamethonium bromide hydrate is an inhibitor of AChR α3 [1-3]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | ~285 °C (dec.) |
|---|---|
| Molecular Formula | C12H30Br2N2 |
| Molecular Weight | 362.188 |
| Exact Mass | 360.077545 |
| Storage condition | Desiccate at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R22;R24/25 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | BQ8575000 |
|
~25% 
Hexamethonium b... CAS#:55-97-0 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 18, # 9 p. 2998 - 3003 |
|
~% 
Hexamethonium b... CAS#:55-97-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 6, p. 402 - 405 |
|
~% 
Hexamethonium b... CAS#:55-97-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 6, p. 402 - 405 |
|
~% 
Hexamethonium b... CAS#:55-97-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 6, p. 402 - 405 |
|
~% 
Hexamethonium b... CAS#:55-97-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 6, p. 402 - 405 |
|
~% 
Hexamethonium b... CAS#:55-97-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 6, p. 402 - 405 |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
A potentially novel nicotinic receptor in Aplysia neuroendocrine cells.
J. Neurophysiol. 112(2) , 446-62, (2014) Nicotinic receptors form a diverse group of ligand-gated ionotropic receptors with roles in both synaptic transmission and the control of excitability. In the bag cell neurons of Aplysia, acetylcholin... |
|
|
Changes in Nicotinic Neurotransmission during Enteric Nervous System Development.
J. Neurosci. 35 , 7106-15, (2015) Acetylcholine-activating pentameric nicotinic receptors (nAChRs) are an essential mode of neurotransmission in the enteric nervous system (ENS). In this study, we examined the functional development o... |
| N,N,N,N',N',N'-Hexamethyl-1,6-hexanediaminium dibromide |
| N,N,N,N',N',N'-hexamethylhexane-1,6-diaminium dibromide |
| Hexamethonium bromide,N,N,N,N',N',N'-Hexamethylhexamethylenediammoniumdibromide |
| Hexamethonium bromide |
| MFCD00011787 |
| 1,6-Hexanediaminium, N,N,N,N',N',N'-hexamethyl-, dibromide |
| Hexamethylenebis(trimethylammonium bromide) |
| 1,6-Hexanediaminium, N,N,N,N',N',N'-hexamethyl-, dibromide (9CI) |
| N,N,N,N',N',N'-Hexamethylhexamethylenediammonium dibromide |
| EINECS 200-249-7 |
| 1,6-Hexanediaminium, N,N,N,N,N,N-hexamethyl-, bromide (1:2) |
| Hexane-1,6-bis(trimethylammonium bromide) |
| Hexamethonium (Bromide) |

![trimethyl-[(E)-6-(trimethylazaniumyl)hex-3-enyl]azanium,dibromide structure](https://image.chemsrc.com/caspic/495/66967-77-9.png)