Sodium levothyroxine pentahydrate
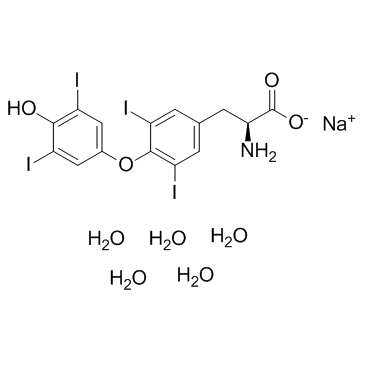
Sodium levothyroxine pentahydrate structure
|
Common Name | Sodium levothyroxine pentahydrate | ||
|---|---|---|---|---|
| CAS Number | 6106-07-6 | Molecular Weight | 888.928 | |
| Density | 2.381 | Boiling Point | N/A | |
| Molecular Formula | C15H20I4NNaO9 | Melting Point | 207-210 (dec.)(lit.) | |
| MSDS | USA | Flash Point | N/A | |
Use of Sodium levothyroxine pentahydrateL-Thyroxine sodium salt pentahydrate (Levothyroxine; T4) is a synthetic hormone in the treatment of hypothyroidism. DIO enzymes convert biologically active thyroid hormone (Triiodothyronine,T3) from L-Thyroxine (T4). |
| Name | L-Thyroxine sodium salt pentahydrate |
|---|---|
| Synonym | More Synonyms |
| Description | L-Thyroxine sodium salt pentahydrate (Levothyroxine; T4) is a synthetic hormone in the treatment of hypothyroidism. DIO enzymes convert biologically active thyroid hormone (Triiodothyronine,T3) from L-Thyroxine (T4). |
|---|---|
| Related Catalog | |
| Target |
Thyroid Hormone Receptor |
| In Vivo | Deiodinases (DIOs), which catalyse the conversion of thyroxine (pro-hormone) to the active thyroid hormone, are associated with thyroid stimulating hormone (TSH) levels. DIO1 and DIO2 catalyze activation of thyroid hormone secretion in contrast to DIO3 playing role inactivation of the secretion. Activities of DIO1 and DIO2 play pivotal role in the negative feedback regulation of pituitary TSH secretion[1]. L-Thyroxine (T4) and Triiodothyronine (T3) hormones are known to modulate the expression of ionic channels, pumps and regulatory contractile proteins. Moreover, thyroid hormones have been shown to influence calcium homeostasis and flux responsible for excitation and contractility, with L-Thyroxine and Triiodothyronine modulating its pharmacological control and secretion. In rats fed 12 weeks with the iodine-free diet, a significant decrease in the levels of both Triiodothyronine and L-Thyroxine is observed when compared to the control group fed with standard diet (p<0.001). In the group treated with low doses of L-Thyroxine, an increase in L-Thyroxine levels is observed (p=0.02) while Triiodothyronine levels remain virtually similar to the control group (p=0.19). Rats treated with high doses of L-Thyroxine display a significant increase in both Triiodothyronine and L-Thyroxine circulating concentrations compared to the non-treated hypothyroid group (p<0.001 and p=0.004, respectively) and a significant increase in L-Thyroxine levels when compared to the control values (p=0.03)[2]. |
| Animal Admin | Rats[2] Sprague-Dawley female rats (N=22) are used. Non-pregnant rats are divided into four groups: 1) control, 2) hypothyroidism, 3) hypothyroidism treated with low doses of L-Thyroxine (20 μg/kg/day) and 4) with high doses of L-Thyroxine (100 μg/kg/day). Control rats (group 1) are fed with standard diet, while the intervention rats are fed with iodine-free diet for 12 weeks to induce hypothyroidism (groups 2-4) which is continued for four more weeks to allow screening of hypothyroid status and L-Thyroxine-treatment. Food and water (iodine-free diet) are available ad libitum. The hypothyroid group treated with low (group 3) or high doses of L-Thyroxine (group 4) are injected intraperitoneally every 24 h with respectively 20 μg/kg/day and 100 μg/kg/day. Blood samples are collected for thyroid function screening at week 12 and 16 following the initiation of either the control or iodine-free diet. Hysterectomy is performed under general anesthesia (isoflurane 2%) at the end of the treatment and the two uterine horns are placed in physiological Krebs' solution until isometric tension measurements within no more than 1 h. |
| References |
| Density | 2.381 |
|---|---|
| Melting Point | 207-210 (dec.)(lit.) |
| Molecular Formula | C15H20I4NNaO9 |
| Molecular Weight | 888.928 |
| Exact Mass | 888.721436 |
| PSA | 141.76000 |
| LogP | 3.60140 |
| Appearance of Characters | powder |
| Storage condition | 2-8°C |
| Water Solubility | cell culture medium: 0.1 mg/mL |
Synonym:L-Thyroxine, Sodium Salt, Pentahydrate; L-3,3',5,5'-Tetraiodothyronine sodium salt; Eferox, Laevoxin; Letter; Levaxin; Levothroid Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 20/21 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Harmful by inhalation and in contact with skin. Potential Health Effects Eye: May cause severe eye irritation. Skin: May cause skin irritation. Ingestion: May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May cause thyroid abnormalities. Ingestion increases the metabolic rate causing warm, flushed and moist skin, muscular weakness, rapid heart rate, insomnia, nervousness, increased metabolism and weight loss. Effects may be delayed. Inhalation: May cause respiratory tract irritation. May cause effects similar to those described for ingestion. Chronic: Chronic exposure can lead to iodism characterized by salivation, nasal discharge, sneezing, conjunctivitis, fever, laryngitis, bronchitis, stomatitis, and skin rashes. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid if irritation develops or persists. Wash clothing before reuse. Flush skin with plenty of soap and water. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Dusts at sufficient concentrations can form explosive mixtures with air. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Extinguishing Media: Use alcohol foam, carbon dioxide, or water spray when fighting fires involving this material. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Storage: Store in a cool, dry place. Hormones and antibiotics room. Keep containers tightly closed. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 6106-07-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: beige grey Odor: Odorless. pH: Not available. Vapor Pressure: Neglilible. Viscosity: Not available. Boiling Point: Not applicable. Freezing/Melting Point: Decomposes. Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: 207-210 deg C Solubility in water: Soluble in water. Specific Gravity/Density: 2.381 Molecular Formula: C15H10I4NO4Na.5H2O Molecular Weight: 888.7491 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, excess heat, oxidizers. Incompatibilities with Other Materials: Explosion hazard when mixed with strong oxidizers. Hazardous Decomposition Products: Nitrogen oxides, carbon monoxide, carbon dioxide, hydrogen iodide, iodide ions (I-). Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 6106-07-6: YP2833760 LD50/LC50: Not available. Carcinogenicity: L-Thyroxine, Sodium Salt Pentahydrate - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.* Hazard Class: 6.1 UN Number: 2811 Packing Group: III IMO Shipping Name: TOXIC SOLID, ORGANIC, N.O.S. Hazard Class: 6.1 UN Number: 2811 Packing Group: III RID/ADR Shipping Name: TOXIC SOLID, ORGANIC, N.O.S. Hazard Class: 6.1 UN Number: 2811 Packing group: III Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: XN Risk Phrases: R 20/21 Harmful by inhalation and in contact with skin. Safety Phrases: S 23 Do not inhale gas/fumes/vapour/spray. WGK (Water Danger/Protection) CAS# 6106-07-6: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 6106-07-6 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 6106-07-6 is not listed on the TSCA inventory. It is for research and development use only. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | 40 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | YP2833760 |
|
Inducible, tightly regulated and growth condition-independent transcription factor in Saccharomyces cerevisiae.
Nucleic Acids Res. 42(17) , e130, (2014) The precise control of gene expression is essential in basic biological research as well as in biotechnological applications. Most regulated systems available in yeast enable only the overexpression o... |
|
|
Replisome-mediated translesion synthesis and leading strand template lesion skipping are competing bypass mechanisms.
J. Biol. Chem. 289(47) , 32811-23, (2014) A number of different enzymatic pathways have evolved to ensure that DNA replication can proceed past template base damage. These pathways include lesion skipping by the replisome, replication fork re... |
|
|
Mirolase, a novel subtilisin-like serine protease from the periodontopathogen Tannerella forsythia.
Biol. Chem. 396(3) , 261-75, (2015) The genome of Tannerella forsythia, an etiological factor of chronic periodontitis, contains several genes encoding putative proteases. Here, we characterized a subtilisin-like serine protease of T. f... |
| MFCD00149110 |
| EINECS 200-221-4 |
| Sodium (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoate hydrate (1:1:5) |
| L-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-, sodium salt, hydrate (1:1:5) |
| sodium,(2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoate,pentahydrate |
| L-Thyroxine (sodium salt pentahydrate) |