Oxiracetam
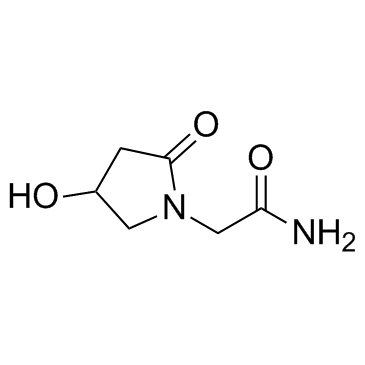
Oxiracetam structure
|
Common Name | Oxiracetam | ||
|---|---|---|---|---|
| CAS Number | 62613-82-5 | Molecular Weight | 158.155 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 494.6±40.0 °C at 760 mmHg | |
| Molecular Formula | C6H10N2O3 | Melting Point | 165-168ºC | |
| MSDS | Chinese USA | Flash Point | 252.9±27.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of OxiracetamOxiracetam is a cyclic derivative of γ-aminobutyric acid (GABA) which has been commonly used as nootropic drug to treat cognitive impairments. |
| Name | 4-Hydroxy-2-Oxopyrrolidine-N-Acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | Oxiracetam is a cyclic derivative of γ-aminobutyric acid (GABA) which has been commonly used as nootropic drug to treat cognitive impairments. |
|---|---|
| Related Catalog | |
| Target |
GABA receptor[1] |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 494.6±40.0 °C at 760 mmHg |
| Melting Point | 165-168ºC |
| Molecular Formula | C6H10N2O3 |
| Molecular Weight | 158.155 |
| Flash Point | 252.9±27.3 °C |
| Exact Mass | 158.069138 |
| PSA | 83.63000 |
| LogP | -2.48 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.570 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UX9656638 |
| HS Code | 2933790090 |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |
|
Minaprine, but not oxiracetam, prevents desipramine-induced impairment of avoidance learning in mice.
Pol. J. Pharmacol. 47(1) , 69-73, (1995) The tricyclic antidepressant desipramine impaired shuttle-box avoidance acquisition in mice of the CD-1 strain. The nootropic drug oxiracetam was unable to prevent the desipramine-induced learning imp... |
|
|
Pre- and postprandial pyridostigmine and oxiracetam effects on growth hormone secretion in anorexia nervosa.
Psychoneuroendocrinology 21(7) , 621-9, (1996) Previous studies have shown that food ingestion is not capable of inhibiting the GHRH-induced GH release in anorexia nervosa, at variance with what is observed in normal subjects. Moreover, a choliner... |
|
|
Putative cognition enhancers reverse kynurenic acid antagonism at hippocampal NMDA receptors.
Eur. J. Pharmacol. 272(2-3) , 203-9, (1995) Oxiracetam, aniracetam and D-cycloserine, three putative cognition enhancers, were examined in a functional assay for NMDA receptors. Rat hippocampal slices or synaptosomes were labeled with [3H]norad... |
| 4-Hydroxy-2-oxopyrrolidine-N-acetamide |
| 2-(4-Hydroxypyrrolidin-2-on-1-yl)acetamide |
| MFCD00242951 |
| oxiracetam |
| 1-Pyrrolidineacetamide, 4-hydroxy-2-oxo- |
| 2-(4-hydroxy-2-oxopyrrolidin-1-yl)acetamide |
| 2-(4-Hydroxy-2-oxo-1-pyrrolidinyl)acetamide |
| UNII:P7U817352G |
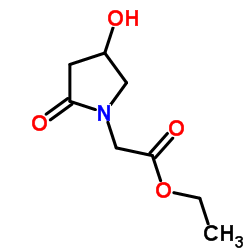 CAS#:62613-81-4
CAS#:62613-81-4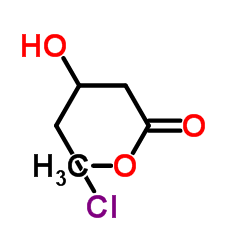 CAS#:10488-68-3
CAS#:10488-68-3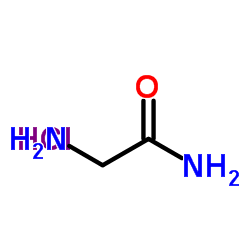 CAS#:1668-10-6
CAS#:1668-10-6 CAS#:4509-09-5
CAS#:4509-09-5 CAS#:154522-71-1
CAS#:154522-71-1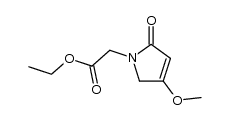 CAS#:110104-61-5
CAS#:110104-61-5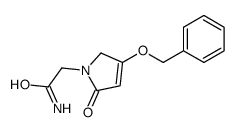 CAS#:113896-94-9
CAS#:113896-94-9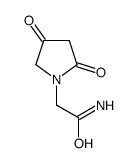 CAS#:85614-54-6
CAS#:85614-54-6
