1-Adamantanamine hydrochloride
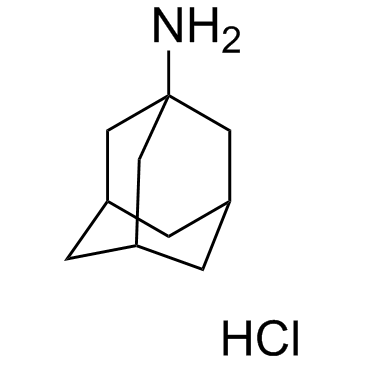
1-Adamantanamine hydrochloride structure
|
Common Name | 1-Adamantanamine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 665-66-7 | Molecular Weight | 187.710 | |
| Density | 1.067g/cm3 | Boiling Point | 225.7ºC at 760 mmHg | |
| Molecular Formula | C10H18ClN | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 96ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 1-Adamantanamine hydrochlorideAmantadine Hydrochloride is an antiviral and an antiparkinsonian drug.Target: Influenza VirusAmantadine is an antiviral that is used in the prophylactic or symptomatic treatment of influenza A. It is also used as an antiparkinsonian agent, to treat extrapyramidal reactions, and for postherpetic neuralgia. Amantadine binding of M2, based on studies of a peptide representing the M2 transmembrane segment in dodecylphosphocholine micelles. Amantadine competes with protons for binding to the deprotonated tetramer, thereby stabilizing the tetramer in a slightly altered conformation. This model accounts for the observed inhibition of proton flux by amantadine [1]. In contrast to most other described channel-blocking molecules, amantadine causes the channel gate of NMDA receptors to close more quickly. Amantadine binding inhibits current flow through NMDA receptor channels but show that its main inhibitory action at pharmaceutically relevant concentrations results from stabilization of closed states of the channel [2]. |
| Name | amantadine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Amantadine Hydrochloride is an antiviral and an antiparkinsonian drug.Target: Influenza VirusAmantadine is an antiviral that is used in the prophylactic or symptomatic treatment of influenza A. It is also used as an antiparkinsonian agent, to treat extrapyramidal reactions, and for postherpetic neuralgia. Amantadine binding of M2, based on studies of a peptide representing the M2 transmembrane segment in dodecylphosphocholine micelles. Amantadine competes with protons for binding to the deprotonated tetramer, thereby stabilizing the tetramer in a slightly altered conformation. This model accounts for the observed inhibition of proton flux by amantadine [1]. In contrast to most other described channel-blocking molecules, amantadine causes the channel gate of NMDA receptors to close more quickly. Amantadine binding inhibits current flow through NMDA receptor channels but show that its main inhibitory action at pharmaceutically relevant concentrations results from stabilization of closed states of the channel [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.067g/cm3 |
|---|---|
| Boiling Point | 225.7ºC at 760 mmHg |
| Melting Point | >300 °C(lit.) |
| Molecular Formula | C10H18ClN |
| Molecular Weight | 187.710 |
| Flash Point | 96ºC |
| Exact Mass | 187.112778 |
| PSA | 26.02000 |
| LogP | 3.41620 |
| Index of Refraction | 1.558 |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S22-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AU4375000 |
| HS Code | 2921300090 |
|
~97% 
1-Adamantanamin... CAS#:665-66-7 |
| Literature: Hays, David S.; Fu, Gregory C. Journal of Organic Chemistry, 1998 , vol. 63, # 9 p. 2796 - 2797 |
|
~82% 
1-Adamantanamin... CAS#:665-66-7 |
| Literature: Tsutsui, Hironori; Ichikawa, Tomoko; Narasaka, Koichi Bulletin of the Chemical Society of Japan, 1999 , vol. 72, # 8 p. 1869 - 1878 |
|
~% 
1-Adamantanamin... CAS#:665-66-7 |
| Literature: Organic Letters, , vol. 11, # 2 p. 433 - 436 |
|
~% 
1-Adamantanamin... CAS#:665-66-7 |
| Literature: WO2007/96124 A1, ; Page/Page column 15-16 ; |
|
~10% 
1-Adamantanamin... CAS#:665-66-7 |
| Literature: Huard, Kim; Lebel, Helene Chemistry - A European Journal, 2008 , vol. 14, # 20 p. 6222 - 6230 |
|
~% 
1-Adamantanamin... CAS#:665-66-7 |
| Literature: Journal of Organic Chemistry, , vol. 45, # 26 p. 5239 - 5243 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2921300090 |
|---|---|
| Summary | 2921300090 other cyclanic, cyclenic or cyclotherpenic mono- or polyamines, and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
CDDP supramolecular micelles fabricated from adamantine terminated mPEG and β-cyclodextrin based seven-armed poly (L-glutamic acid)/CDDP complexes.
Colloids Surf. B Biointerfaces 105 , 31-6, (2013) This research is aimed to develop a nano-sized supramolecular micelle delivery system of cis-dichlorodiammine platinum (II) (CDDP) in order to achieve the passive tumor targeting. Firstly, star-shaped... |
|
|
In vitro and in vivo identification of ABCB1 as an efflux transporter of bosutinib.
J. Hematol. Oncol. 8 , 81, (2015) Bosutinib is a recently approved ABL inhibitor. In spite of the well-documented effectiveness of BCR-ABL inhibitors in treating chronic myeloid leukemia, development of resistance is a continuous clin... |
|
|
Platinum-Incorporating Poly(N-vinylpyrrolidone)-poly(aspartic acid) Pseudoblock Copolymer Nanoparticles for Drug Delivery.
Biomacromolecules 16 , 2059-71, (2015) Cisplatin-incorporating pseudoblock copolymer nanoparticles with high drug loading efficiency (ca. 50%) were prepared built on host-guest inclusion complexation between β-cyclodextrin end-capped poly(... |
| Amazolon |
| adamantanamine hydrochloride |
| Amantadine HCl |
| Lysovir |
| EINECS 211-560-2 |
| MFCD00074723 |
| virasol |
| 1-aminoadamantane hydrochloride |
| Virofral |
| 1-Adamantanaminehydrochloride |
| Mantadix |
| 1-adamantamine hydrochloride |
| Midantan |
| 1-Adamantanamine hydrochloride |
| Adekin |
| Mantadan |
| 1-adamantylamine hydrochloride |
| exp105-1 |
| Amantadine hydrochloride |
| Amantadine (hydrochloride) |



 CAS#:3717-60-0
CAS#:3717-60-0 CAS#:4411-25-0
CAS#:4411-25-0 CAS#:5689-59-8
CAS#:5689-59-8 CAS#:22110-53-8
CAS#:22110-53-8 CAS#:458529-20-9
CAS#:458529-20-9 CAS#:2387-23-7
CAS#:2387-23-7 CAS#:274901-16-5
CAS#:274901-16-5 CAS#:702-82-9
CAS#:702-82-9![Urea,N-tricyclo[3.3.1.13,7]dec-1-yl- structure](https://image.chemsrc.com/caspic/034/13072-69-0.png) CAS#:13072-69-0
CAS#:13072-69-0
