Teprenone
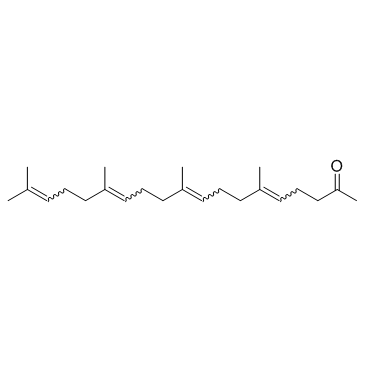
Teprenone structure
|
Common Name | Teprenone | ||
|---|---|---|---|---|
| CAS Number | 6809-52-5 | Molecular Weight | 330.55 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 442.2±24.0 °C at 760 mmHg | |
| Molecular Formula | C23H38O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 168.0±9.8 °C | |
Use of TeprenoneTeprenone is a anti-ulcer drug, and works as an inducer of heat shock proteins (HSPs). |
| Name | teprenone |
|---|---|
| Synonym | More Synonyms |
| Description | Teprenone is a anti-ulcer drug, and works as an inducer of heat shock proteins (HSPs). |
|---|---|
| Related Catalog | |
| Target |
HSP |
| In Vitro | Teprenone is an inducer of HSPs. Teprenone (Geranylgeranylacetone, 1 μM) significantly prevents ethanol-induced exfoliation, and reduces lactate dehydrogenase (LDH) release in gastric mucosal cells. Teprenone (1 μM) gradually increases HSC70 level, and rapidly accumulates the stress-inducible HSP90, HSP70, and HSP60 concentrations within 30-60 min. Teprenone also activates the heat shock factor 1[1]. Teprenone (0-20 µM) slightly increases human umbilical vein endothelial cell (HUVEC) viability following irradiation (IR). Teprenone (10 µM) exhibits no effects on HUVEC migration and invasion, but enhances HUVEC tube formation and wound healing both with and without IR. Teprenone (10 µM) also promotes angiogenesis by inducing VEGF and eNOS expression in HUVECs[3]. |
| In Vivo | Teprenone (200 mg/kg, p.o.) results in the accumulation of HSP70 mRNA in rats, and the accumulation is enhanced by stress addition in the mucosa of Teprenone-pretreated rats compared with that of vehicle-pretreated rats. Teprenone (200 mg/kg, p.o.) markedly suppresses the ulcer formation after 2- and 4-hour stress loading in rats[1]. Teprenone (200 mg/kg daily) induces HSP72 in retinal ganglion cells (RGCs) from rat retinas. Teprenone significantly reduces the loss of RGCs (evaluated after intraocular pressure (IOP) elevation), lessens optic nerve damage, decreases the number of TUNEL-positive cells in the RGC layer, and increases HSP72 in a rat model of glaucoma[2]. Teprenone (200 mg/kg, p.o.) shows protective effect on radiation-induced intestinal injury in mice[3]. |
| Cell Assay | Human umbilical vein endothelial cells (HUVECs) are seeded onto 48-well plates at a density of 1 × 102 cells/well before Teprenone and/or radiation treatment. Cell viability is determined at 48 h after treatment using 0.5 mg/mL MTT solution in serum-free media. This solution is incubated with the cells for 2 h in the 37°C humidified atmosphere containing 5% CO2. Then, the MTT solution is removed, and the cells are dissolved in 100 µL of DMSO. Optical densities of the supernatants are measured at 540 nm with an ELISA spectrophotometer[3]. |
| Animal Admin | Male Wister strain rats weighing approximately 250 g are individually housed in wire-mesh cages in a room maintained at 23°C on a 12-hour light-dark cycle. Rats are allowed free access to a standard laboratory chow. Teprenone (200 mg/kg; as emulsion with 5% gum arabic and 0.008% α-tocopherol) or vehicle (5% gum arabic emulsion containing 0.008% α-tocopherol) is given orally in a volume of 5 mL/kg through a metal tubing attached to a 6-mL syringe. Two hours later, rats are placed in restraint cages and then vertically immersed in water at 23°C to the level of the xyphoid process. The rats are killed by decapitation at the indicated times[1]. |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 442.2±24.0 °C at 760 mmHg |
| Molecular Formula | C23H38O |
| Molecular Weight | 330.55 |
| Flash Point | 168.0±9.8 °C |
| PSA | 17.07000 |
| LogP | 8.20 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.483 |
| Storage condition | ?20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| RTECS | RA5391300 |
| HS Code | 2914190090 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
| HS Code | 2914190090 |
|---|---|
| Summary | 2914190090 other acyclic ketones without other oxygen function。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Heat shock protein 72 suppresses apoptosis by increasing the stability of X-linked inhibitor of apoptosis protein in renal ischemia/reperfusion injury.
Mol. Med. Report. 11(3) , 1793-9, (2014) X‑linked inhibitor of apoptosis protein (XIAP) negatively regulates apoptotic pathways at a post‑mitochondrial level. XIAP functions by directly binding and inhibiting activation of specific caspases.... |
|
|
Aldioxa improves delayed gastric emptying and impaired gastric compliance, pathophysiologic mechanisms of functional dyspepsia.
Sci. Rep. 5 , 17519, (2015) Delayed gastric emptying and impaired gastric accommodation (decreased gastric compliance) play important roles in functional dyspepsia (FD). Here we screen for a clinically used drug with an ability ... |
|
|
Efficacy of omeprazole, famotidine, mosapride and teprenone in patients with upper gastrointestinal symptoms: an omeprazole-controlled randomized study (J-FOCUS).
BMC Gastroenterol. 12 , 42, (2012) In Japan, treatment guidelines are lacking for patients with upper gastrointestinal symptoms. We aimed to compare the efficacy of different drugs for the treatment of uninvestigated upper gastrointest... |
| 5,9,13,17-Nonadecatetraen-2-one, 6,10,14,18-tetramethyl-, (E,E,E)- |
| Geranylgeranyl acetone |
| GGA |
| 5,9,13,17-Nonadecatetraen-2-one, 6,10,14,18-tetramethyl-, (5E,9E,13E)- |
| geranylgeranylacetone |
| 6,10,14,18-Tetramethyl-5,9,13,17-nonadecatetraen-2-one |
| Teprenone |
| (5E,9E,13E)-6,10,14,18-Tetramethyl-5,9,13,17-nonadecatetraen-2-one |
| (5E,9E,13E)-6,10,14,18-tetramethylnonadeca-5,9,13,17-tetraen-2-one |
| E36U31 |
| MFCD00866901 |
| Selbex |
 CAS#:71668-81-0
CAS#:71668-81-0 CAS#:1206897-24-6
CAS#:1206897-24-6 CAS#:1694-31-1
CAS#:1694-31-1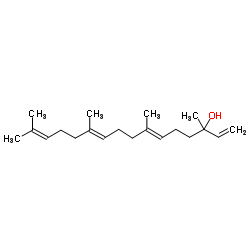 CAS#:1113-21-9
CAS#:1113-21-9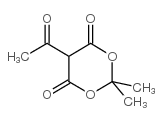 CAS#:72324-39-1
CAS#:72324-39-1 CAS#:186581-53-3
CAS#:186581-53-3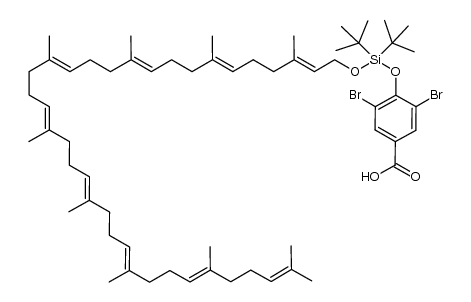 CAS#:1033756-53-4
CAS#:1033756-53-4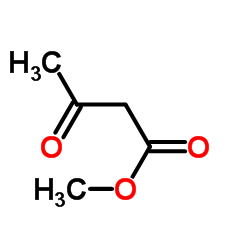 CAS#:105-45-3
CAS#:105-45-3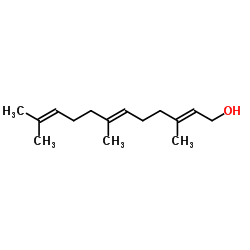 CAS#:106-28-5
CAS#:106-28-5 CAS#:1182-68-9
CAS#:1182-68-9