pemirolast
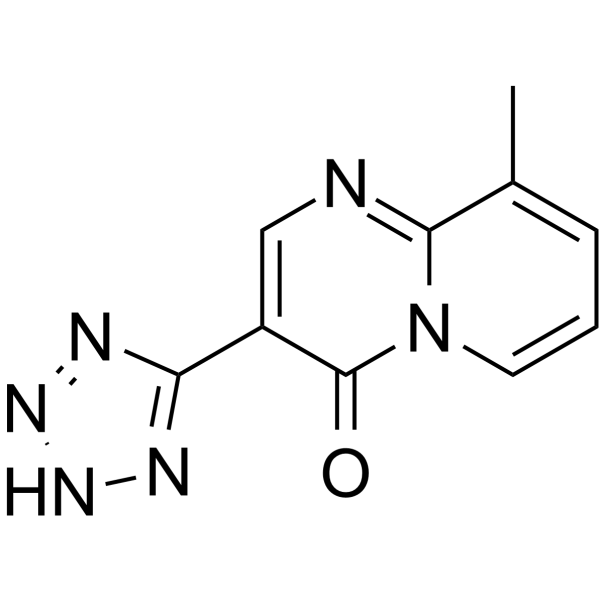
pemirolast structure
|
Common Name | pemirolast | ||
|---|---|---|---|---|
| CAS Number | 69372-19-6 | Molecular Weight | 228.21000 | |
| Density | 1.64 g/cm3 | Boiling Point | 454.8ºC at 760 mmHg | |
| Molecular Formula | C10H8N6O | Melting Point | 310-311ºC (decomposes) | |
| MSDS | N/A | Flash Point | 228.9ºC | |
Use of pemirolastPemirolast is an orally active antiallergic agent. Pemirolast attenuates paclitaxel hypersensitivity reactions, can be used for bronchial asthma and conjunctivitis research[1]-[5]. |
| Name | 9-methyl-3-(2H-tetrazol-5-yl)pyrido[1,2-a]pyrimidin-4-one |
|---|---|
| Synonym | More Synonyms |
| Description | Pemirolast is an orally active antiallergic agent. Pemirolast attenuates paclitaxel hypersensitivity reactions, can be used for bronchial asthma and conjunctivitis research[1]-[5]. |
|---|---|
| Related Catalog | |
| In Vitro | Pemirolast (1 μM-1 mM) inhibits A23187-induced LTC4 and ECP release from the eosinophils in a dose-dependent manner[1]. Pemirolast (0.1 mM and 1 mM) also inhibits PAF-induced and FMLP-induced ECP release from the eosinophils[1]. Pemirolast prevents the activation of human eosinophils to inhibit granule protein LTQ and ECP release, so that alleviates controlling allergic diseases[1]. Pemirolast (100 nM-1 mM; 1-15 min) fails to significantly inhibit histamine release from human conjunctival mast cells[2]. Pemirolast (0.1 μg/mL-0.01 mg/mL) inhibits the activation of signal transduction phospholipases C and AZ in rat peritoneal mast cells, by inhibiting the degranulation reaction of antigen and compound 48/80, suppressing the formation of 1,2-diacylglycerol and phosphatidylic acid[3]. |
| In Vivo | Pemirolast potently attenuates paclitaxel hypersensitivity reactions through inhibition of the release of sensory neuropeptides in rats[4]. Pemirolast (0.1-1 mg/kg; i.v.) inhibits taxel-induced pulmonary vascular hyperpermeability, and reverses paclitaxel-induced arterial PaO2 decreasing at a dosage of 1 mg/kg, 30 minutes after paclitaxel injection (15 mg/kg; i.v.)[4]. Pemirolast (1 mg/kg; i.v.) reverses taxel-induced elevation of the concentrations of sensory neuropeptides (CGRP, substance P and neurokinin A), 30 minutes after paclitaxel injection (15 mg/kg; i.v.)[4]. Pemirolast (10 mg/kg/d; p.o.; 4-5 d) significantly reduces cisplatin-induced kaolin intake on days 3 and 4 and inhibits cisplatin-induced substance P release in the cerebrospinal fluid (CSF) in rats[5]. Animal Model: Male Wistar rats (6-week-old, 160-250 g)[5] Dosage: 10 mg/kg Administration: Oral gavage; 5 days: 1 h or 30 min before and 24, 48, 72 and 96 h (five times in total) after administration of cisplatin (2-10 mg/kg; i.v.) Result: Inhibited the cisplatin-induced increase in kaolin intake on days 3 and 4, without decreasing in normal feed intake. Animal Model: Male Wistar rats (6-week-old, 160-250 g)[5] Dosage: 10 mg/kg Administration: Oral gavage; 4 days: 30 min before and 24, 48, 72 and 96 h (four times in total) after administration of cisplatin (5 mg/kg; i.v.). Result: Significantly reversed the cisplatin-induced increase of substance P levels to vehicle levels in the CSF. |
| References |
| Density | 1.64 g/cm3 |
|---|---|
| Boiling Point | 454.8ºC at 760 mmHg |
| Melting Point | 310-311ºC (decomposes) |
| Molecular Formula | C10H8N6O |
| Molecular Weight | 228.21000 |
| Flash Point | 228.9ºC |
| Exact Mass | 228.07600 |
| PSA | 88.83000 |
| LogP | 0.18300 |
| Hazard Codes | C |
|---|---|
| Risk Phrases | R34:Causes burns. |
| Safety Phrases | 16-26-36/37/39-37/39 |
| RIDADR | UN 1993 3/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 8 |
|
~74% 
pemirolast CAS#:69372-19-6 |
| Literature: Wako Pure Chemical Industries, Ltd.; Tokyo, Tanabe Co., Ltd. Patent: US5081243 A1, 1992 ; |
|
~89% 
pemirolast CAS#:69372-19-6 |
| Literature: Wako Pure Chemical Industries, Ltd.; Tokyo, Tanabe Co., Ltd. Patent: US5081243 A1, 1992 ; |
|
~% 
pemirolast CAS#:69372-19-6 |
| Literature: Sano, Atsunori; Ishihara, Masami Heterocycles, 1998 , vol. 48, # 4 p. 775 - 778 |
| 3-(1H-tetrazol-5-yl)-9-methyl-4-oxo-4H-pyrido[1,2-a]pyrimidine |
| Pemirolast (INN) |
| UNII-2C09NV773M |
| Pemirox (TN) |
| Pemirolastum [INN-Latin] |
| Pemiroplast Potassium |
| MFCD00864611 |
| Pemirolastum |
| Pemirolast |
| 9-methyl-3-(1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyrimidin-4-one |
| 9-TBX |
![4-Imino-9-methyl-4H-pyrido[1,2-a]pyrimidine-3-carbonitrile structure](https://image.chemsrc.com/caspic/364/102781-19-1.png)
![9-Methyl-3-(1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyrimidin-4-imine structure](https://image.chemsrc.com/caspic/182/132056-88-3.png)

 CAS#:100299-08-9
CAS#:100299-08-9