KUL 7211 racemate
Modify Date: 2025-08-27 12:34:28
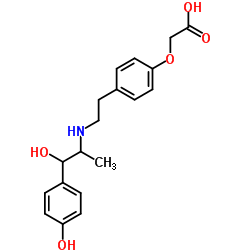
KUL 7211 racemate structure
|
Common Name | KUL 7211 racemate | ||
|---|---|---|---|---|
| CAS Number | 911196-40-2 | Molecular Weight | 345.390 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 600.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C19H23NO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 317.2±30.1 °C | |
Use of KUL 7211 racemateKUL 7211 racemate is the racemate of KUL 7211. KUL 7211 is a selective β-adrenoceptor agonist. |
| Name | KUL 7211 racemate |
|---|---|
| Synonym | More Synonyms |
| Description | KUL 7211 racemate is the racemate of KUL 7211. KUL 7211 is a selective β-adrenoceptor agonist. |
|---|---|
| Related Catalog | |
| Target |
β-adrenoceptor[1] |
| In Vitro | KUL 7211 (KUL-7211) is a new β-adrenoceptor agonist in vitro. In rat isolated organs, its selectivities, for inhibition of spontaneous uterine contraction (mediated via β2-adrenergic stimulation) and inhibition of colonic contraction (via β3-adrenergic stimulation) versus increase in atrial rate (via β1-adrenergic stimulation), are 56.3 and 242.2, respectively. KUL 7211 relaxes 80 mM KCl induced tonic contractions in both rabbit (pD2 value: 5.86±0.13, whose ureteral relaxation is mediated via β2-adrenergic stimulation) and canine (pD2 value: 6.52±0.16, via β3-adrenergic stimulation) isolated ureters in a concentration-dependent manner. These KUL 7211-induced relaxing effects are antagonized by ICI-118,551 (selective β2-adrenoceptor antagonist, pKB value: 8.91±0.24) in the rabbit ureter and by bupranolol (non-selective β-adernoceptor antagonist, pKB value: 6.85±0.12) in the canine ureter. KUL 7211 also reduces the spontaneous rhythmic contraction in a canine ureteral spiral preparation in a concentration-dependent manner, the pD2 value being 6.83±0.20. These data clearly demonstrate that KUL 7211 selectively stimulates both ureteral β2- and β3-adrenoceptors and potently relaxes ureteral smooth muscle[1]. |
| Kinase Assay | KUL 7211 (KUL-7211) is dissolved in distilled water with an equivalent molarity of HCl[1]. Experiments are carried out using rat isolated atria, uterus, and proximal colon. Rats are stunned and then killed by rapid exsanguination. Each organ is rapidly removed and suspended in a 10 or 20 mL organ bath. The bath solution is Krebs solution (118.1 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4• 7H2O, 25.0 mM NaHCO3, 1.2 mM KH2PO4, 11.1 mM glucose) in the atria and colon experiments and Locke-Ringer solution (154 mM NaCl, 5.6 mM KCl, 2.6 mM CaCl2, 2.1 mM MgCl2, 6.0 mM NaHCO3, 2.8 mM glucose) in the uterus experiment. Each is continuously gassed with a mixture of 95% oxygen and 5% carbon dioxide at 37°C. The bath solutions contain 1 μM phentolamine, 0.5μM desipramine, and 3 0μ M hydrocortisone to block α-adrenoceptors and neuronal and extraneuronal catecholamine uptake, respectively. Spontaneous contractions are measured isometrically by means of a force-transducer and measuring system (TB-611T, AP-601G, and RPM-6004 or model 45196A, model 1829, and model 7903) connected to a thermowriting rectigraph. An initial resting tension of 5 mN is placed on each organ, and it is allowed to equilibrate for 1 h. Each preparation is exposed to only one drug[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 600.9±50.0 °C at 760 mmHg |
| Molecular Formula | C19H23NO5 |
| Molecular Weight | 345.390 |
| Flash Point | 317.2±30.1 °C |
| Exact Mass | 345.157623 |
| LogP | 1.47 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.604 |
| [4-(2-{[1-Hydroxy-1-(4-hydroxyphenyl)-2-propanyl]amino}ethyl)phenoxy]acetic acid |
| Acetic acid, 2-[4-[2-[[2-hydroxy-2-(4-hydroxyphenyl)-1-methylethyl]amino]ethyl]phenoxy]- |